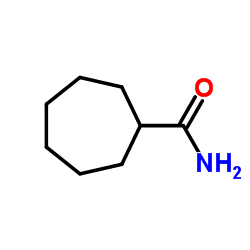
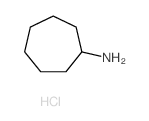
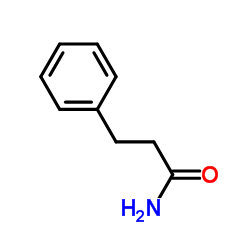
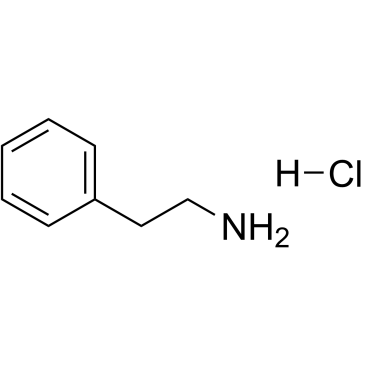
|
~93% |
|
~90% |
|
~99% |
|
~84% |
|
~80% |
|
~90% |
|
~84% |
|
~89% |
|
~80% |
|
~92% |
|
~83% |
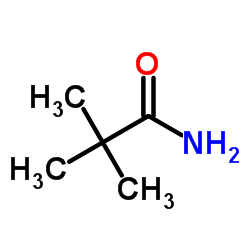
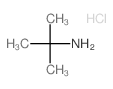
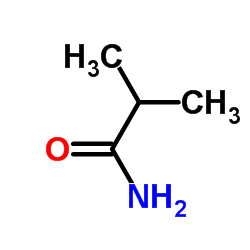

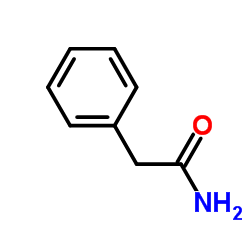
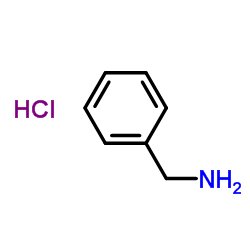
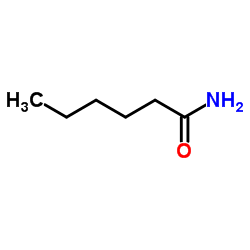
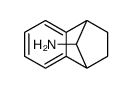

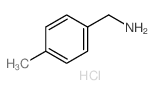

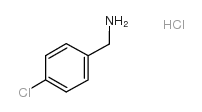
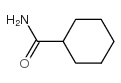
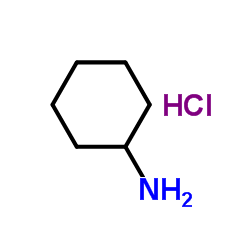
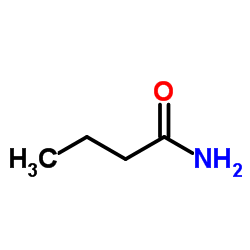

![[4-(trifluoromethyl)phenyl]acetamide Structure](https://image.chemsrc.com/caspic/248/41360-55-8.png)