| Structure | Name/CAS No. | Articles |
|---|---|---|
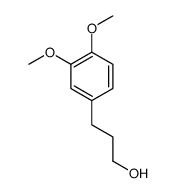 |
3-(3,4-dimethoxyphenyl)propan-1-ol
CAS:3929-47-3 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
3-(3,4-dimethoxyphenyl)propan-1-ol
CAS:3929-47-3 |