Inactivation of aromatase in vitro by 4-hydroxy-4-androstene-3,17-dione and 4-acetoxy-4-androstene-3,17-dione and sustained effects in vivo.
A M Brodie, W M Garrett, J R Hendrickson, C H Tsai-Morris, P A Marcotte, C H Robinson
Index: Steroids 38(6) , 693-702, (1981)
Full Text: HTML
Abstract
4-Hydroxy-4-androstene-3,17-dione (4-OHA) and 4-acetoxy-4-androstene-3,17-dione (4-AcA), in addition to being competitive inhibitors of aromatase, cause time-dependent, irreversible, loss of enzyme activity in both human placental and rat ovarian microsomes. In vivo, treatment of rats with 4-OHA also causes loss of ovarian aromatase activity. To test whether this loss of activity could have in vivo significance, rats with hormone-dependent, mammary tumors were treated with 4-OHA on alternate weeks. Tumor regression continued to occur during the weeks without treatment. These findings suggest that inactivation of aromatase is important in the mechanism of action of the compounds in vivo.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
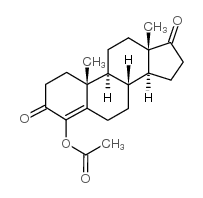 |
4-Androsten-4-ol-3,17-dione acetate
CAS:61630-32-8 |
C21H28O4 |
|
Aromatase inhibitors and the treatment of breast cancer.
1986-01-01 [J. Steroid Biochem. 24 , 91, (1986)] |
|
Inhibition of estrogen biosynthesis and regression of mammar...
1981-01-01 [Adv. Exp. Med. Biol. 138 , 179-90, (1981)] |
|
Overview of recent development of aromatase inhibitors.
1982-08-01 [Cancer Res. 42(8 Suppl) , 3312s-3314s, (1982)] |
|
Effects of aromatase inhibitor 4-hydroxyandrostenedione and ...
1982-08-01 [Cancer Res. 42(8 Suppl) , 3360s-3364s, (1982)] |
|
Aromatase enzyme catalysis is involved in the potent inhibit...
1982-01-01 [Mol. Pharmacol. 21(1) , 173-80, (1982)] |