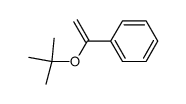
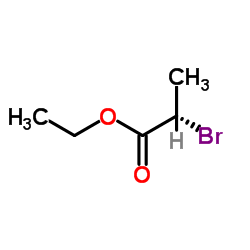
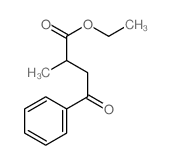
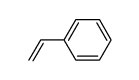
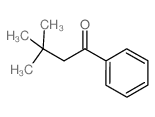
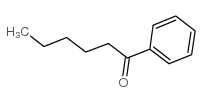
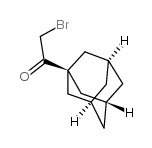
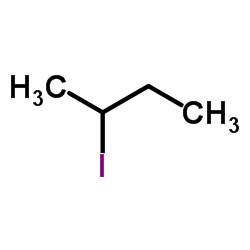
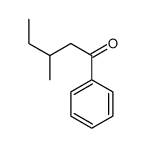
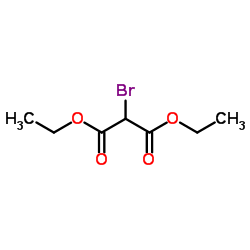
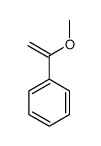
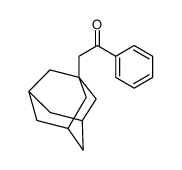
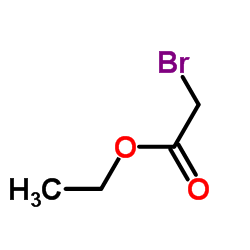
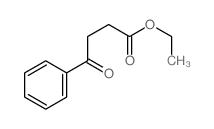
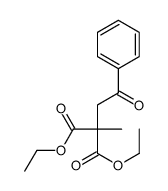
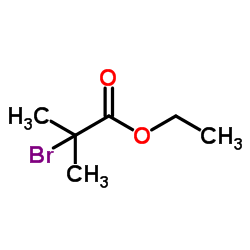
|
~84% |
|
~% |
|
~95% |
|
~81% |
|
~% |
|
~% |
|
~% |
|
~83% |
|
~% |
|
~79% |
|
~93% |
|
~0% |
|
~4% |
|
~0% |
|
~% |
|
~% |
|
~83% |
|
~88% |
|
~% |
|
~85% |
|
~83% |