The pH and anion effects on the heterogeneous photocatalytic degradation of o-methylbenzoic acid in TiO2 aqueous suspension.
K H Wang, Y H Hsieh, C H Wu, C Y Chang
Index: Chemosphere 40(4) , 389-94, (2000)
Full Text: HTML
Abstract
This investigation used UV light of 365 nm and titanium dioxide in aqueous suspension to study the photocatalytic reaction of o-methylbenzoic acid under the influence of pH values, anion additives and the varieties of titanium dioxide. From experimental results, under the condition of 5 g/l TiO2, pH 3 and light intensity of 2.45 mW/cm2, 0.1 mM of o-methylbenzoic acid could be completely decomposed in 2 h. The reaction was faster with lowering pH, and was found to be apparent first-order following Langmuir-Hinshelwood model. In the presence of anion additives, the inhibitive effect of chloride ions was larger than that of sulfate ions under acidic condition for Degussa brand titanium dioxide, but without influence using Janssen brand. Both brands, however, promoted the mineralization of o-methylbenzoic acid (o-MBA).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
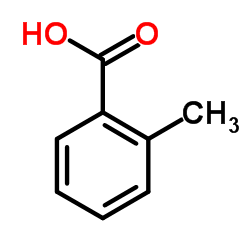 |
2-Methylbenzoic acid
CAS:118-90-1 |
C8H8O2 |
|
Effect of aspirin treatment on the prevention of esophageal ...
2014-06-01 [Oncol. Rep. 31(6) , 2785-91, (2014)] |
|
Protozoan ALKBH8 oxygenases display both DNA repair and tRNA...
2014-01-01 [PLoS ONE 9(6) , e98729, (2014)] |
|
Oxidation of tolualdehydes to toluic acids catalyzed by cyto...
1995-02-01 [Drug Metab. Dispos. 23(2) , 261-5, (1995)] |
|
Effects of the headspace gas composition on anaerobic biotra...
2003-06-01 [J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng. 38(6) , 1099-113, (2003)] |
|
Dual-opposite injection electrokinetic chromatography for th...
2000-06-01 [Electrophoresis 21(10) , 1997-2009, (2000)] |