Enantioselective fluorination of organic molecules. I. Synthetic studies of the agents for electrophilic, enantioselective fluorination of carbanions.
Y Takeuchi, A Satoh, T Suzuki, A Kameda, M Dohrin, T Satoh, T Koizumi, K L Kirk
Index: Chem. Pharm. Bull. 45(6) , 1085-8, (1997)
Full Text: HTML
Abstract
In order to develop novel methods for electrophilic and enantioselective fluorination of active methine compounds, preliminary experiments were carried out. The N-tosyl derivative 5 obtained from D-phenylglycine was fluorinated with FClO3 or diluted F2 gas to give the N-fluoro-N-tosyl derivative 6. N-tosyl- or N-mesyl-(S)-alpha-phenethylamine 7 or 8 was subjected to FClO3 fluorination to produce the corresponding N-fluoro derivative, 10 or 11, respectively. Enantioselective fluorination of some methine compounds was attempted employing the above N-fluoro agents. Best result was obtained when 2-benzyl-1-tetralone/KHMDS was treated with 10 to produce the fluorinated tetralone 17 in 53% yield with enantiomeric excess (ee) of 48%.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
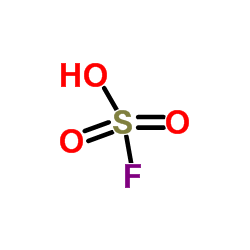 |
Fluorosulfuric acid
CAS:7789-21-1 |
FHO3S |
|
Synthesis of alpha-fluorosulfonate and alpha-fluorosulfonami...
2006-11-23 [Org. Lett. 8(24) , 5617-20, (2006)] |
|
Tailoring of interfacial mechanical shear strength by surfac...
2015-05-26 [ACS Nano 9 , 5143-53, (2015)] |
|
Perfluoro Allyl fluorosulfate (FAFS): a versatile building b...
2011-01-01 [Molecules 16 , 6512, (2011)] |
|
Stable ion NMR and GIAO-DFT study of novel cations from 8,16...
2008-01-18 [J. Org. Chem. 73(2) , 457-66, (2008)] |
|
Adenosinetriphosphate sulfurylase from Penicillium chrysogen...
1985-08-01 [Arch. Biochem. Biophys. 240(2) , 509-23, (1985)] |