| Structure | Name/CAS No. | Articles |
|---|---|---|
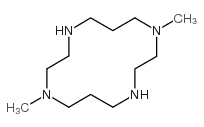 |
1,3-DIHYDRO-4-(5-FLUORO-2-HYDROXYPHENYL)-2H-1,5-BENZODIAZEPIN-2-ONE
CAS:214078-92-9 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
1,3-DIHYDRO-4-(5-FLUORO-2-HYDROXYPHENYL)-2H-1,5-BENZODIAZEPIN-2-ONE
CAS:214078-92-9 |