| Structure | Name/CAS No. | Articles |
|---|---|---|
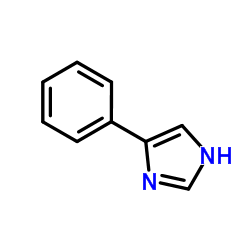 |
4-PHENYLIMIDAZOLE
CAS:670-95-1 |
|
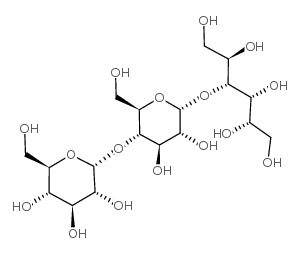 |
MALTOTRIITOL
CAS:32860-62-1 |