| Structure | Name/CAS No. | Articles |
|---|---|---|
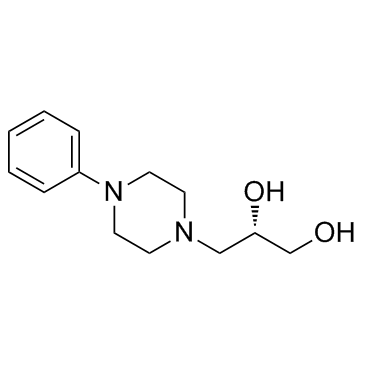 |
Levodropropizine
CAS:99291-25-5 |
|
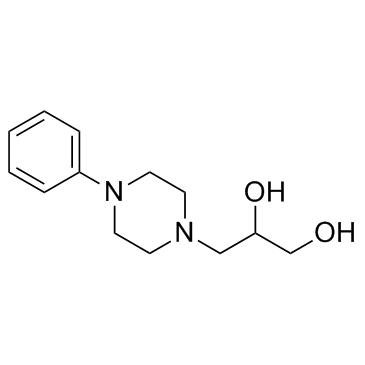 |
Dropropizine
CAS:17692-31-8 |