Selective boron-containing thrombin inhibitors--X-ray analysis reveals surprising binding mode.
A von Matt, C Ehrhardt, P Burkhard, R Metternich, M Walkinshaw, C Tapparelli
Index: Bioorg. Med. Chem. 8(9) , 2291-303, (2000)
Full Text: HTML
Abstract
Based on the structural comparison of the S1 pocket in different trypsin-like serine proteases, a series of Boc-D-trimethylsilylalanine-proline-boro-X pinanediol derivatives, with boro-X being different amino boronic acids, have been synthesized as inhibitors of thrombin. Among the novel compounds, a number of derivatives were synthesized which appeared to have side-chain variants too big to fit into the S1 pocket. Nevertheless, these compounds inhibited thrombin in the nM range. The X-ray structure of one of these inhibitors bound to the active side of thrombin reveals that a new binding mode is responsible for these surprising results.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
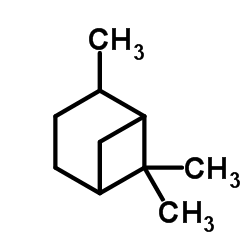 |
Pinane
CAS:4795-86-2 |
C10H18 |
|
Metabolic profiling of GuanXin II prescription based on meta...
2011-03-25 [J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011)] |
|
Chemistry around pinene and pinane: a facile synthesis of cy...
2008-06-01 [Chem. Biodivers. 5(6) , 910-9, (2008)] |
|
Acute toxicities to larval rainbow trout of representative c...
1991-02-01 [Bull. Environ. Contam. Toxicol. 46(2) , 173-8, (1991)] |
|
Role of thromboxane and angiotensin in cyclosporine-induced ...
1993-01-01 [J. Heart Lung Transplant. 12(5) , 851-5, (1993)] |
|
Terpenoids biotransformation in mammals III: Biotransformati...
1981-04-01 [J. Pharm. Sci. 70(4) , 406-15, (1981)] |