Enzyme conformational dynamics during catalysis and in the 'resting state' monitored by hydrogen/deuterium exchange mass spectrometry.
Yu-Hong Liu, Lars Konermann
Index: FEBS Lett. 580(22) , 5137-42, (2006)
Full Text: HTML
Abstract
This work reports the use of electrospray mass spectrometry for studying the conformational dynamics of enzymes by amide hydrogen/deuterium exchange (HDX) measurements. A rapid-mixing quench-flow approach allows comparisons to be made between the HDX kinetics of free enzymes with those under steady-state conditions. Experiments carried out on carboxypeptidase B in the absence of substrate and in the presence of saturating concentrations of hippuryl-Arg result in HDX kinetics that are indistinguishable. This finding implies that the conformational dynamics that mediate HDX are not significantly different in the resting state of the enzyme and during substrate turnover.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
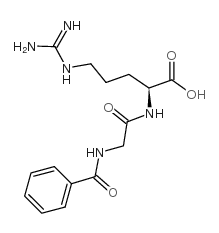 |
Hippuryl-Arg-OH
CAS:744-46-7 |
C15H21N5O4 |
|
High-level secretory expression of human procarboxypeptidase...
2008-12-01 [J. Microbiol. Biotechnol. 18(12) , 1938-44, (2008)] |
|
Assay of carboxypeptidase N activity in serum by liquid-chro...
1985-12-01 [Clin. Chem. 31(12) , 1936-9, (1985)] |
|
Model studies on protein glycation: influence of cysteine on...
2008-04-01 [Ann. N. Y. Acad. Sci. 1126 , 248-52, (2008)] |
|
Alterations of carboxypeptidases N activities in patients wi...
1987-02-01 [Clin. Biochem. 20(1) , 43-6, (1987)] |
|
Biochemical characterization of bovine plasma thrombin-activ...
2009-01-01 [BMC Biochem. 10 , 13, (2009)] |