A simple spectrophotometric assay of carboxypeptidase N (kininase I) in human serum.
H Schweisfurth, E Reinhart, J Heinrich, E Brugger
Index: J. Clin. Chem. Clin. Biochem. 21(10) , 605-9, (1983)
Full Text: HTML
Abstract
Kininase I (carboxypeptidase N; EC 3.4.17.3) consists of carboxypeptidase N1 (CN1) and carboxypeptidase N2 (CN2); these two enzymes can be differentiated by their activities towards hippuryl-L-arginine and hippuryl-L-lysine, respectively. A spectrophotometric assay for both carboxypeptidases in human serum is described and the biochemical behaviour of these enzymes investigated. The pH optima are found to be 8.4 for CN1 and CN2. The Michaelis-Menten constants are: CN1 4.59 +/- 0.03 mmol/l; CN2 37.26 +/- 3.49 mmol/l. CN2 can be inhibited by EDTA (76%), dimercaprolum (97%) and phenanthroline (98%). Diisopropylfluorophosphate has no influence on both enzymes. Elevated haemoglobin only interferes with CN1 measurements, and high bilirubin concentrations slightly alter the activity of both enzymes. High CN1 activities were found in sera of patients with sarcoidosis, and elevated CN2 activities were found in lung cancer.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
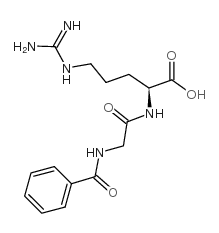 |
Hippuryl-Arg-OH
CAS:744-46-7 |
C15H21N5O4 |
|
High-level secretory expression of human procarboxypeptidase...
2008-12-01 [J. Microbiol. Biotechnol. 18(12) , 1938-44, (2008)] |
|
Assay of carboxypeptidase N activity in serum by liquid-chro...
1985-12-01 [Clin. Chem. 31(12) , 1936-9, (1985)] |
|
Model studies on protein glycation: influence of cysteine on...
2008-04-01 [Ann. N. Y. Acad. Sci. 1126 , 248-52, (2008)] |
|
Alterations of carboxypeptidases N activities in patients wi...
1987-02-01 [Clin. Biochem. 20(1) , 43-6, (1987)] |
|
Biochemical characterization of bovine plasma thrombin-activ...
2009-01-01 [BMC Biochem. 10 , 13, (2009)] |