Enhancement of solubility by temporary dimethoxybenzyl-substitution of peptide bonds. Towards the synthesis of defined oligomers of alanine and of lysyl-glutamyl-glycine.
J Blaakmeer, T Tijsse-Klasen, G I Tesser
Index: Int. J. Pept. Protein Res. 37(6) , 556-64, (1991)
Full Text: HTML
Abstract
The synthesis of the model compound Aloc-Ala-Ala-Dma-Ala-Ala-OMe has been described as an illustration of the fact that a large group reversibly alkylating the amido group of an oligomer can disturb the regularity of a peptide backbone, oppose its aggregation and thus enhance its solubility greatly, affording synthons for further oligomerization. Application of such a group not only affects the solubility, but alters also the properties of the intermediates. The concomitant change in reactivity may run to such an extent that N-alkylation of oligomers has to be abandoned (this was encountered in the attempted synthesis of Lys-Glu-Dmg). Consequently, the solubility of the growing protected peptide chain will become progressively less and in the mentioned example the oligomerization had to be terminated at the dodecapeptide level, indicating the severe need for reversible "structure-breaking" functions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
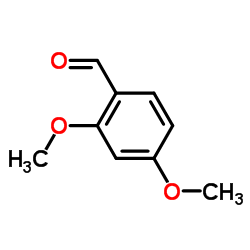 |
2,4-dimethoxybenzaldehyde
CAS:613-45-6 |
C9H10O3 |
|
Antifungal activity of redox-active benzaldehydes that targe...
2011-01-01 [Ann. Clin. Microbiol. Antimicrob. 10 , 23, (2011)] |
|
Rational design of inhibitors of VirA-VirG two-component sig...
2007-06-15 [Bioorg. Med. Chem. Lett. 17 , 3281-6, (2007)] |
|
Can phlorotannins purified extracts constitute a novel pharm...
2012-01-01 [PLoS ONE 7(2) , e31145, (2012)] |
|
Anti-proliferative activity and chemical characterization by...
2016-01-08 [J. Chromatogr. A. 1428 , 115-25, (2016)] |
|
The ortho backbone amide linker (o-BAL) is an easily prepare...
2002-01-01 [J. Comb. Chem. 4(3) , 223-8, (2002)] |