Characterization of the single Ca(2+)-binding site on the Ca(2+)-ATPase reconstituted with short- or long-chain phosphatidylcholines.
A P Starling, Y M Khan, J M East, A G Lee
Index: Biochem. J. 304 ( Pt 2) , 569-75, (1994)
Full Text: HTML
Abstract
On reconstitution of the Ca(2+)-ATPase of skeletal muscle sarcoplasmic reticulum into bilayers of dimyristoleoylphosphatidylcholine [di(C14:1)PC] or dinervonylphosphatidylcholine [di(C24:1)PC] the stoichiometry of Ca2+ binding changes from the usual two Ca2+ ions bound per ATPase molecule to one Ca2+ ion bound per ATPase molecule. For the ATPase in di(C24:1)PC, removal of Ca2+ from the Ca(2+)-bound ATPase results in a decrease in tryptophan fluorescence intensity, as observed for the ATPase in dioleoylphosphatidylcholine [di(C18:1)PC]. For the ATPase in di(C14:1)PC removal of Ca2+ results in no change in tryptophan fluorescence intensity. In the presence of Mg2+, removal of Ca2+ from the ATPase in di(C18:1)PC or di(C24:1)PC results in a decrease in tryptophan fluorescence intensity, but for the ATPase in di(C14:1)PC this results in an increase in intensity. Fluorescence of the ATPase labelled with 4-nitrobenzo-2-oxa-1,3-diazole (NBD) is the same for the ATPase in di(C18:1)PC or di(C24:1)PC, but is markedly greater in di(C14:1)PC, consistent with a 4-fold increase in the E1/E2 equilibrium constant. Addition of Mg2+ to NBD-labelled ATPase in di(C18:1) PC or di(C24:1)PC results in an increase in NBD fluorescence, attributed to stronger binding of Mg2+ to the E1 than to the E2 conformation; addition of Mg2+ had no effect on the fluorescence of the NBD-labelled ATPase in di(C14:1)PC. In the absence of Ca2+, Mg2+ increased the tryptophan fluorescence of the ATPase in di(C14:1)PC, di(C18:3)PC or di(C24:1)PC, with the same binding-constant for Mg2+ in all three lipids. Addition of Mg2+ to the ATPase labelled with 4-(bromomethyl)-6,7-dimethoxycoumarin resulted in a decrease in fluorescence in di(C18:1)PC or di(C24:1)PC but had no effect in di(C14:1)PC. These effects are interpreted in terms of binding of Ca2+ at a single outer Ca2+ binding-site on the ATPase in di(C14:1)PC and di(C24:1)PC, in a conformation in which the inner site is occluded [in di(C14:1)PC] or modified in its affinity for Ca2+ [in di(C24:1)PC]. Thapsigargin binds to the ATPase, reducing its affinity for Ca2+ both in di(C14:1)PC and di(C24:1)PC.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
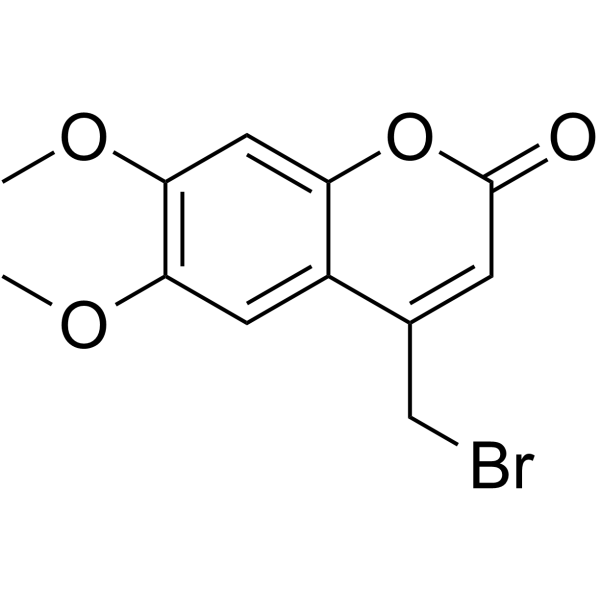 |
4-(Bromomethyl)-6,7-dimethoxy-2H-chromen-2-one
CAS:88404-25-5 |
C12H11BrO4 |
|
Simultaneous radiometric and fluorimetric detection of lauri...
1996-06-07 [J. Chromatogr. B, Biomed. Appl. 681(2) , 233-9, (1996)] |
|
Labeling of immunoglobulins with bifunctional, sulfhydryl-se...
1993-01-01 [Bioconjug. Chem. 4(4) , 300-4, (1993)] |
|
Investigation of derivatization reagents for the analysis of...
1994-01-01 [Nat. Toxins 2(5) , 302-11, (1994)] |
|
Labeling the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum ...
1992-07-07 [Biochemistry 31(26) , 6023-31, (1992)] |
|
Simultaneous determination of mycophenolic acid and valproic...
2006-04-01 [Biomed. Chromatogr. 20(4) , 319-26, (2006)] |