Histidine-rich glycoprotein: a possible modulator of coagulation and fibrinolysis.
Sadao Wakabayashi, Takehiko Koide
Index: Semin. Thromb. Hemost. 37 , 389-394, (2011)
Full Text: HTML
Abstract
Histidine-rich glycoprotein (HRG) is one of the major plasma proteins and thought to function in blood coagulation, fibrinolysis, and innate immune systems. The amino acid sequence of HRG revealed a multidomain structure consisting of cystatin-like domains 1 and 2, a Pro-rich domain 1, a His-rich domain, a Pro-rich domain 2, and a C-terminal domain. Broad ligand-binding properties of HRG are involved in the multivalent functions of HRG. Among various functions of HRG, its interactions with heparin/heparan sulfate, fibrinogen, and plasminogen are thought to be intimately related to its roles in the coagulation and fibrinolytic systems. Recent studies on these topics are mainly reviewed in this article. The newly disclosed abilities of HRG in angiogenesis, its antibacterial effect, its activation of T-cell lines in cooperation with Concanavalin A, and the identification of a putative receptor for HRG on T cell lines are also described.© Thieme Medical Publishers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
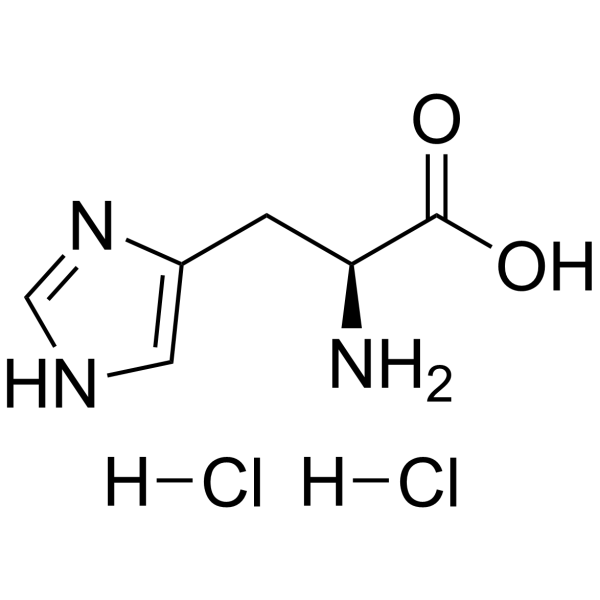 |
Histidine
CAS:6027-02-7 |
C6H10ClN3O2 |
|
Highly specific in vivo gene delivery for p53-mediated apopt...
2015-01-01 [Nat. Commun. 6 , 6456, (2015)] |
|
Histidine kinases and response regulators in networks.
2012-04-01 [Curr. Opin. Microbiol. 15(2) , 118-24, (2011)] |
|
Assessing the fractions of tautomeric forms of the imidazole...
2011-04-05 [Proc. Natl. Acad. Sci. U. S. A. 108 , 5602-5607, (2011)] |
|
Could carnosine or related structures suppress Alzheimer's d...
2007-05-01 [J. Alzheimers Dis. 11 , 229-240, (2007)] |
|
Influence of amino acid relative position on the oxidative m...
2011-03-01 [Anal. Bioanal. Chem 399 , 2779-2794, (2011)] |