Synthesis of C-substituted t-BuNH-8,9-R,R'-nido-7,8,9-C3B8H9 (R,R' = H,H; MeH; Me,Me; Ph,H and Ph,Ph) tricarbollide compounds and their tautomeric conversions. Effect of substituents on tautomeric equilibria between neutral and zwitterionic forms.
Mario Bakardjiev, Josef Holub, Bohumil Stíbr, Ivana Císarová
Index: Dalton Trans. 39(17) , 4186-90, (2010)
Full Text: HTML
Abstract
Treatment of C-substituted nido dicarbadecaboranes 5,6-R',R-5,6-C(2)B(8)H(10) (1) (where R',R = H,H (1a); H,Me, (1b); Me,Me, (1c); H,Ph, (1d) and Ph,Ph, (1e) with 1,8-bis-(dimethylamino)naphthalene (proton sponge = PS) and t-BuNC in CH(2)Cl(2), followed by acidification, generated a series of pure neutral compounds 7-t-BuNH-8,9-R,R'-nido-7,8,9-C(3)B(8)H(9) (N2) (where R,R' = H,H (N2a); H,Me (N2b); Me,Me (N2c); H,Ph (N2d), and Ph,Ph (N2e)), each of which exhibits tautomerism. Dissolution of the substituted compounds (N2b-N2e) in protic solvents (PRS), such as MeCN and Me(2)CO, leads to tautomeric equilibrium with the zwitterionic tautomers 7-t-BuNH(2)-8,9-R,R'-nido-7,8,9-C(3)B(8)H(8) (Z2) (where R,R'= H,H (Z2a); H,Me (Z2b); Me,Me (Z2c); H,Ph (Z2d) and Ph,Ph (Z2e)), while the unsubstituted compound N2a exhibits absolute tautomerism--a complete conversion into the zwitterionic tautomer Z2a. The tautomeric behaviour of individual compounds is therefore strongly affected by the nature of the substituent, as assessed via NMR spectroscopy in terms of tautomerisation constants K(T) = C(Z2)/C(N2) (where C(Z2) and C(N2) are equilibrium concentrations of Z2 and N2 forms in a given solvent). Individual tautomers were characterised by (11)B and (1)H NMR spectroscopy and the structure of the monomethylated N2b tautomer was determined by an X-ray diffraction study.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
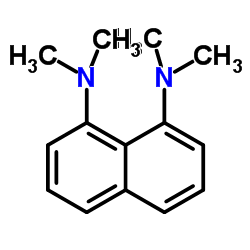 |
1,8-bis(dimethylamino)naphthalene
CAS:20734-58-1 |
C14H18N2 |
|
In situ characterizing membrane lipid phenotype of breast ca...
2015-01-01 [Sci. Rep. 5 , 11298, (2015)] |
|
Distribution study of atorvastatin and its metabolites in ra...
2014-07-01 [Anal. Bioanal. Chem 406(19) , 4601-10, (2014)] |
|
Proton sponge: a novel and versatile MALDI matrix for the an...
2009-10-01 [Anal. Chem. 81 , 7954-7959, (2009)] |
|
Lipid fingerprinting of gram-positive lactobacilli by intact...
2011-06-30 [Rapid Commun. Mass Spectrom. 25 , 1757-1764, (2011)] |
|
1,8-Bis(dimethylamino)naphthalene: a novel superbasic matrix...
2009-08-01 [Rapid Commun. Mass Spectrom. 23 , 2380-2382, (2009)] |