New 5alpha-reductase inhibitors: in vitro and in vivo effects.
Víctor Pérez-Ornelas, Marisa Cabeza, Eugene Bratoeff, Ivonne Heuze, Mauricio Sánchez, Elena Ramírez, Elia Naranjo-Rodríguez
Index: Steroids 70(3) , 217-24, (2005)
Full Text: HTML
Abstract
The enzyme 5alpha-reductase is responsible for the conversion of testosterone (T) to its more potent androgen dihydrotestosterone (DHT). This steroid had been implicated in androgen-dependent diseases such as: benign prostatic hyperplasia, prostate cancer, acne and androgenic alopecia. The inhibition of 5alpha-reductase enzyme offers a potentially useful treatment for these diseases. In this study, we report the synthesis and pharmacological evaluation of several new 3-substituted pregna-4, 16-diene-6, 20-dione derivatives. These compounds were prepared from the commercially available 16-dehydropregnenolone acetate. The biological activity of the new steroidal derivatives was determined in vivo as well as in vitro experiments. In vivo experiments, the anti-androgenic effect of the steroids was demonstrated by the decrease of the weight of the prostate gland of gonadectomized hamster treated with T plus finasteride or the new steroids. The IC50 value of these steroids was determined by measuring the conversion of radio labeled T to DHT. The results of this study carried out with 5alpha-reductase enzyme from hamster and human prostate showed that four of the six steroidal derivatives (5, 7, 9, 10) exhibited much higher 5alpha-reductase inhibitory activity, as indicated by the IC50 values than the presently used Proscar 3 (finasteride). The comparison of the weight of the hamster's prostate gland indicated that compound 5 had a comparable weight decrease as finasteride. The overall data of this study showed very clearly those compounds 5, 7, 9, 10 are good inhibitors for the 5alpha-reductase enzyme.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
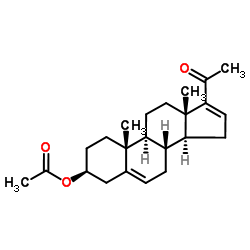 |
16-Dehydropregenolone Acetate
CAS:979-02-2 |
C23H32O3 |
|
[Acylhydrazones of 20-keto steroids and their transformation...
2007-01-01 [Bioorg. Khim. 33(3) , 337-41, (2007)] |
|
Pregna-D'-pentaranes - a new class of active gestagenes.
1982-01-01 [J. Steroid Biochem. 16(1) , 61-7, (1982)] |
|
Metabolism of steroid acetates by Streptomyces albus.
1984-03-01 [J. Steroid Biochem. 20(3) , 781-4, (1984)] |
|
Oxidative side-chain and ring fission of pregnanes by Arthro...
1988-11-01 [Biochem. J. 255(3) , 769-74, (1988)] |