Enantioselective recognitions of chiral molecular tweezers containing imidazoliums for amino acids.
Xiaoyu Su, Kui Luo, Qingxiang Xiang, Jingbo Lan, Rugang Xie
Index: Chirality 21(5) , 539-46, (2009)
Full Text: HTML
Abstract
Two kinds of novel chiral molecular tweezers containing imidazoliums were synthesized from L-alanine, L-phenylalanine, and L-glutamic acid. They are constructed by the chiral imidazolium pincers and two different spacers which are 1,3-bis (bromomethyl)benzene and 2,6-bis(bromomethyl)pyridine, respectively. The enantioselective recognition of L- and D-amino acid derivatives by these molecular tweezers was investigated by UV spectroscopic titration experiments and good enantioselectivities were obtained, which are highly sensitive to whether the spacer has the binding site and the pincers has the other aromatic rings besides imidazolium ring. The host molecular 3b.2PF6- showed remarkable enantioselectivity for N-Boc protected histidine methyl ester, affording K(L)/K(D) of 5.10.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
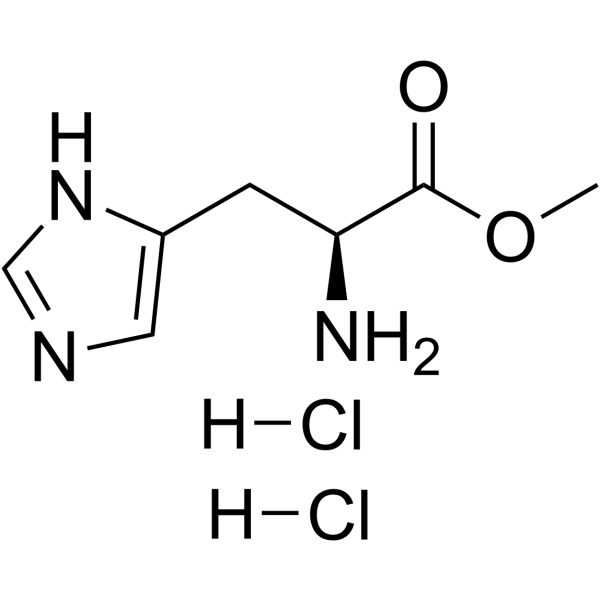 |
H-His-OMe.2HCl
CAS:7389-87-9 |
C7H13Cl2N3O2 |
|
Preparation and use of poly(hydroxyethyl methacrylate) cryog...
2013-02-01 [J. Food Sci. 78(2) , E238-43, (2013)] |
|
A proximal pair of positive charges provides the dominant li...
2012-04-01 [Biochem. J. 443(1) , 65-73, (2012)] |
|
The biological activity of hydrogen peroxide. IV. Enhancemen...
1988-03-01 [Mutat. Res. 198(1) , 233-40, (1988)] |
|
Carbonic anhydrase activators: gold nanoparticles coated wit...
2011-03-10 [J. Med. Chem. 54(5) , 1170-7, (2011)] |
|
Gas-phase structure of protonated histidine and histidine me...
2005-09-22 [J. Phys. Chem. A 109(37) , 8329-35, (2005)] |