Journal of Organic Chemistry
2008-11-07
Kinetic resolution of racemic alcohols using thioamide modified 1-methyl-histidine methyl ester.
Xue-Li Geng, Jia Wang, Guo-Xing Li, Peng Chen, Shu-Fang Tian, Jin Qu
Index: J. Org. Chem. 73(21) , 8558-62, (2008)
Full Text: HTML
Abstract
The low-molecular-weight and easily prepared N-thiobenzoyl 1-methyl-histidine methyl ester 3k was utilized to efficiently catalyze the kinetic resolution of racemic secondary alcohols. Comparison of the conformations of amide catalyst 3c and thioamide catalyst 3k was made to understand the origin of the improvement of the enantioselectivity by thioamide modification.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
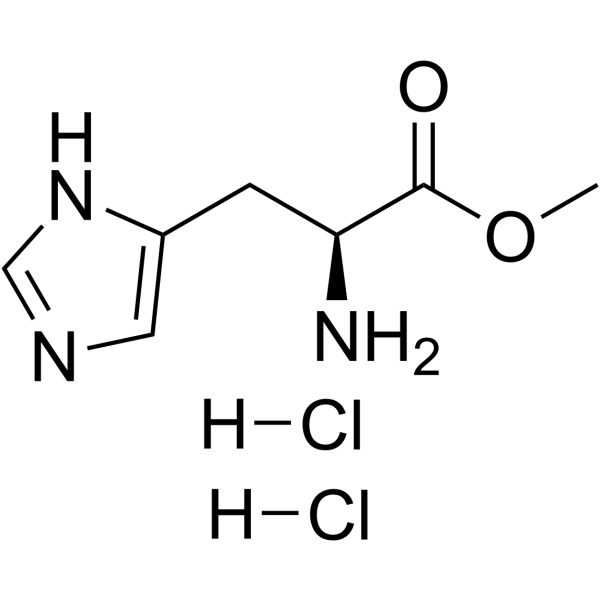 |
H-His-OMe.2HCl
CAS:7389-87-9 |
C7H13Cl2N3O2 |
Related Articles:
More...
|
Preparation and use of poly(hydroxyethyl methacrylate) cryog...
2013-02-01 [J. Food Sci. 78(2) , E238-43, (2013)] |
|
A proximal pair of positive charges provides the dominant li...
2012-04-01 [Biochem. J. 443(1) , 65-73, (2012)] |
|
The biological activity of hydrogen peroxide. IV. Enhancemen...
1988-03-01 [Mutat. Res. 198(1) , 233-40, (1988)] |
|
Carbonic anhydrase activators: gold nanoparticles coated wit...
2011-03-10 [J. Med. Chem. 54(5) , 1170-7, (2011)] |
|
Gas-phase structure of protonated histidine and histidine me...
2005-09-22 [J. Phys. Chem. A 109(37) , 8329-35, (2005)] |