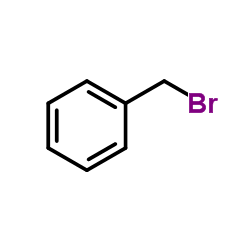
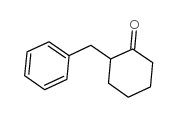
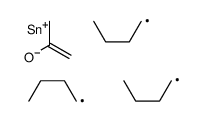
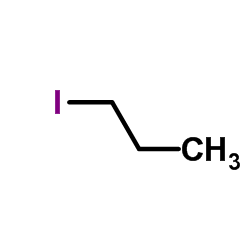
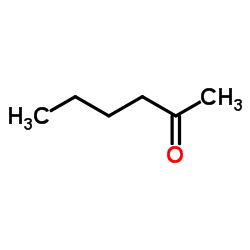
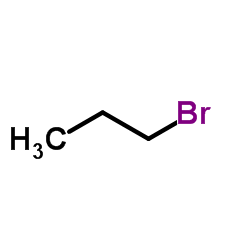
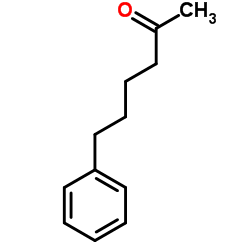
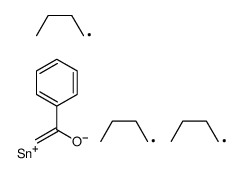
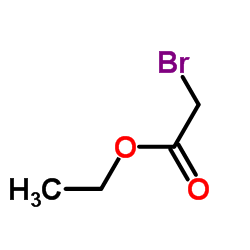
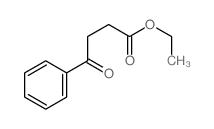
|
~83% |
|
~43% |
|
~32% |
|
~43% |
|
~10% |
|
~56% |