Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
2005-02-01
Chemical behavior of tungstate solutions. Part 1. A spectroscopic survey of the species involved.
Thierry Barré, Laurent Arurault, François X Sauvage
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 61(4) , 551-7, (2005)
Full Text: HTML
Abstract
This study is focused on the composition and the evolution of tungstate ions solutions as a function of pH and increasing concentrations. The Raman analysis showed that, during the titration of the tungstate solutions, WO4(2-), HWO4- ions and probably W2O7(2-) , HW2O7(2-) and H2W2O7 solvated species could exist in aqueous solutions. For diluted solutions, additions of a strong acid does not cause any precipitation, whereas the formation of the unstable solid tungstic acid (H2WO4 or WO3.H2O) could occur in concentrated solutions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
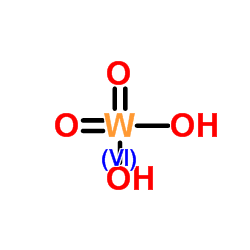 |
Tungstic acid
CAS:7783-03-1 |
H2O4W |
Related Articles:
More...
|
Catalytic conversion of cellulose to ethylene glycol over a ...
2013-04-01 [ChemSusChem 6(4) , 652-8, (2013)] |
|
Identification in various chlorate-resistant mutants of a pr...
1985-04-17 [Biochim. Biophys. Acta 839(2) , 181-90, (1985)] |
|
Effect of oxyanions of the early transition metals on rabbit...
1983-10-11 [Biochemistry 22(21) , 4994-5000, (1983)] |
|
Interaction between isopoly-tungstic acid and berberine hydr...
2003-07-01 [Anal. Sci. 19(7) , 1055-60, (2003)] |
|
Vanadate, tungstate and molybdate activate rod outer segment...
1985-04-22 [Biochim. Biophys. Acta 845(1) , 81-5, (1985)] |