Spectroscopic and DFT study of tungstic acid peroxocomplexes.
Laura Barrio, José M Campos-Martín, José L G Fierro
Index: J. Phys. Chem. A 111(11) , 2166-71, (2007)
Full Text: HTML
Abstract
The catalytic system formed by tungstic acid and its complexes with H2O2 and phenylphosphonic acid has been analyzed from the experimental and theoretical points of view. Previous structural studies by XRD proved the validity of the DFT proposed models and methodology. Hydrogen peroxide reacts with tungstic acid to form a peroxo complex. Vibrational and electronic spectra showed significant changes upon interaction with H2O2. The DFT and TD-DFT for IR and UV-vis calculations not only are in agreement with experimental data but also allow for a deeper characterization of the species formed in in situ conditions. A SCRF/PCM methodology was chosen to account for the solvent effect. The solvent effect of water was considered for geometry re-optimization of the structure and for the TD-DFT calculations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
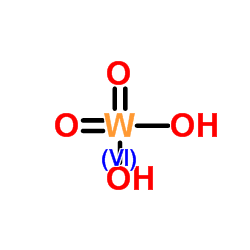 |
Tungstic acid
CAS:7783-03-1 |
H2O4W |
|
Catalytic conversion of cellulose to ethylene glycol over a ...
2013-04-01 [ChemSusChem 6(4) , 652-8, (2013)] |
|
Identification in various chlorate-resistant mutants of a pr...
1985-04-17 [Biochim. Biophys. Acta 839(2) , 181-90, (1985)] |
|
Effect of oxyanions of the early transition metals on rabbit...
1983-10-11 [Biochemistry 22(21) , 4994-5000, (1983)] |
|
Interaction between isopoly-tungstic acid and berberine hydr...
2003-07-01 [Anal. Sci. 19(7) , 1055-60, (2003)] |
|
Vanadate, tungstate and molybdate activate rod outer segment...
1985-04-22 [Biochim. Biophys. Acta 845(1) , 81-5, (1985)] |