A fragmentation study of a flavone triglycoside, kaempferol-3-O-robinoside-7-O-rhamnoside.
Raymond E March, Xiu-Sheng Miao, Chris D Metcalfe
Index: Rapid Commun. Mass Spectrom. 18(9) , 931-4, (2004)
Full Text: HTML
Abstract
A mass spectrometric method based on the combined use of electrospray ionization, collision-induced dissociation and tandem mass spectrometry has been applied to the structural characterization of the flavone triglycoside, robinin (3,5,7,4'-tetrahydroxyflavone-3-O-robinoside-7-O-rhamnoside). The deprotonated molecule fragments by loss of the rhamnose glycan residue to yield the Y(7) (-) ion (m/z 593) and by scission of the robinose glycan residue to yield the radical anion [Y(3,0)-H](-.) (m/z 430). The Y(7) (-) ion fragments by scission of the robinose glycan residue to yield the radical anion of Y(7)[Y(3,0)-H](-.) (m/z 284). The [Y(3,0)-H](-.) radical anion fragments by loss of the rhamnose glycan residue to yield the radical anion Y(7)[Y(3,0)-H](-.) (m/z 284) and by scission to yield [Y(7)-H][Y(3,0)--H](-) (m/z 283). A fragmentation mechanism has been proposed.Copyright 2004 John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
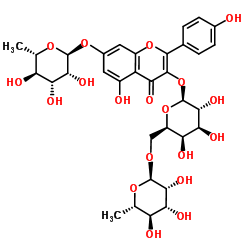 |
Robinin
CAS:301-19-9 |
C33H40O19 |
|
Roles of flavonoids in plant resistance to insects.
1986-01-01 [Prog. Clin. Biol. Res. 213 , 87-100, (1986)] |
|
Simultaneous determination of the flavonoids robinin and kae...
2011-04-28 [J. Pharm. Biomed. Anal. 55 , 109-113, (2011)] |
|
Effects of selected flavonoids and carotenoids on drug accum...
2005-03-01 [In Vivo 19 , 433-8., (2005)] |
|
[Studies on chemical constituents of Artemisia rupestris (II...
2007-06-01 [Zhongguo Zhong Yao Za Zhi 32(12) , 1187-9, (2007)] |
|
[Robinin intoxication].
2002-09-15 [Aten. Primaria 30(4) , 258-9, (2002)] |