Botulinum neurotoxin serotype A inhibitors: small-molecule mercaptoacetamide analogs.
Scott T Moe, Andrew B Thompson, Genessa M Smith, Ross A Fredenburg, Ross L Stein, Alan R Jacobson
Index: Bioorg. Med. Chem. 17(8) , 3072-9, (2009)
Full Text: HTML
Abstract
Botulinum neurotoxin elicits its paralytic activity through a zinc-dependant metalloprotease that cleaves proteins involved in neurotransmitter release. Currently, no drugs are available to reverse the effects of botulinum intoxication. Herein we report the design of a novel series of mercaptoacetamide small-molecule inhibitors active against botulinum neurotoxin serotype A. These analogs show low micromolar inhibitory activity against the isolated enzyme. Structure-activity relationship studies for a series of mercaptoacetamide analogs of 5-amino-3-phenylpyrazole reveal components essential for potent inhibitory activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
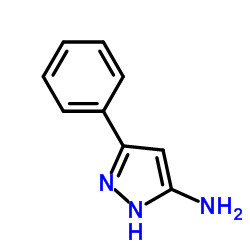 |
3-Phenyl-1H-pyrazol-5-amine
CAS:1572-10-7 |
C9H9N3 |
|
Anthranilamide-pyrazolo[1,5-a]pyrimidine conjugates as p53 a...
2012-08-01 [ChemMedChem 7(8) , 1453-64, (2012)] |
|
An efficient one-step synthesis of heterobiaryl pyrazolo[3,4...
2009-11-19 [Org. Lett. 11(22) , 5214-7, (2009)] |
|
The conversion of isothiazoles into pyrazoles using hydrazin...
[Tetrahedron 65(34) , 7023-7037, (2009)] |
|
Crystal structure of chlorido-tris (3-amino-5-phenyl-1H <...
[Zeitsch. für Kristall. 226(3) , 397-399, (2011)] |
|
Convenient synthesis of some new pyrazolo [5, 1-c] triazines...
[Eur. J. Chem. 3(2) , 129-137, (2012)] |