Poly(ADP-ribose)glycohydrolase is an upstream regulator of Ca2+ fluxes in oxidative cell death.
C Blenn, P Wyrsch, J Bader, M Bollhalder, Felix R Althaus
Index: Cell. Mol. Life Sci. 68 , 1455-66, (2011)
Full Text: HTML
Abstract
Oxidative DNA damage to cells activates poly(ADP-ribose)polymerase-1 (PARP-1) and the poly(ADP-ribose) formed is rapidly degraded to ADP-ribose by poly(ADP-ribose)glycohydrolase (PARG). Here we show that PARP-1 and PARG control extracellular Ca(2+) fluxes through melastatin-like transient receptor potential 2 channels (TRPM2) in a cell death signaling pathway. TRPM2 activation accounts for essentially the entire Ca(2+) influx into the cytosol, activating caspases and causing the translocation of apoptosis inducing factor (AIF) from the inner mitochondrial membrane to the nucleus followed by cell death. Abrogation of PARP-1 or PARG function disrupts these signals and reduces cell death. ADP-ribose-loading of cells induces Ca(2+) fluxes in the absence of oxidative damage, suggesting that ADP-ribose is the key metabolite of the PARP-1/PARG system regulating TRPM2. We conclude that PARP-1/PARG control a cell death signal pathway that operates between five different cell compartments and communicates via three types of chemical messengers: a nucleotide, a cation, and proteins.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
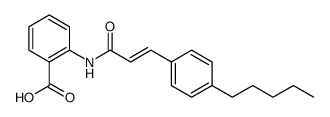 |
N-(P-Amylcinnamoyl)Anthranilic Acid
CAS:99196-74-4 |
C21H23NO3 |
|
Des-Arg9-bradykinin causes kinin B1 receptor mediated endoth...
2014-12-01 [Pharmacol. Res. 90 , 18-24, (2014)] |
|
Enhanced cytotoxicity in triple-negative and estrogen recept...
2015-09-01 [Oncol. Rep. 34 , 1589-98, (2015)] |
|
Diglycolic acid is the nephrotoxic metabolite in diethylene ...
2011-11-01 [Toxicol. Sci. 124 , 35-44, (2011)] |
|
Expression and functional properties of TRPM2 channels in do...
2011-12-01 [J. Neurophysiol. 106 , 2865-75, (2011)] |
|
Differential pathways for calcium influx activated by concan...
2012-02-01 [Pflugers Arch. 463 , 309-18, (2012)] |