Determination of the number of detergent molecules associated with the reaction center protein isolated from the photosynthetic bacterium Rhodopseudomonas viridis. Effects of the amphiphilic molecule 1,2,3-heptanetriol.
P Gast, P Hemelrijk, A J Hoff
Index: FEBS Lett. 337 , 39, (1994)
Full Text: HTML
Abstract
Detergent-free reaction center (RC) proteins from the photosynthetic bacterium Rhodopseudomonas viridis were obtained using Bio-Beads SM-2. With these RCs, the amount of detergent molecules associated with the protein was measured by determining the detergent concentration at which re-solubilization occurred as a function of the RC concentration. For N,N-dimethyl dodecylamine-N-oxide (LDAO), Triton X-100 and beta-octylglucoside 260 +/- 30,105 +/- 10 and 360 +/- 100 detergent molecules were necessary to dissolve the protein, respectively. With this technique we have studied the effect of the amphiphilic molecule 1,2,3-heptanetriol, which is essential in the crystallization process of these RCs. Addition of 5% 1,2,3-heptanetriol reduces the value for LDAO to 120 +/- 20 LDAO/RC, supporting the notion that crystallization of the RCs is promoted by increasing the number of protein-protein contacts.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
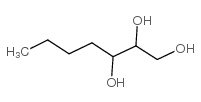 |
1,2,3-heptanetriol
CAS:103404-57-5 |
C7H16O3 |
|
Leukocyte analysis from WHIM syndrome patients reveals a piv...
2008-03-01 [J. Clin. Invest. 118 , 1074-1075, (2008)] |
|
Crystallization of reaction center from Rhodopseudomonas sph...
1984-08-01 [Proc. Natl. Acad. Sci. U. S. A. 81 , 4795, (1984)] |
|
Three-dimensional crystals of a membrane protein complex. Th...
1982-07-05 [J. Mol. Biol. 158 , 567, (1982)] |
|
The association of different detergents with the photosynthe...
1996-08-01 [Eur. J. Biochem. 239(3) , 805-9, (1996)] |
|
X-Ray diffraction analysis of three-dimensional crystals of ...
2000-05-01 [J. Struct. Biol. 130(1) , 73-80, (2000)] |