Confirmation of okadaic acid, dinophysistoxin-1 and dinophysistoxin-2 in shellfish as their anthrylmethyl derivatives using UV radiation.
Dorothea F K Rawn, Cathie Ménard, Barbara Niedzwiadek, David Lewis, Benjamin P Y Lau, Nathalie Delauney-Bertoncini, Marie Claire Hennion, James F Lawrence
Index: J. Chromatogr. A. 1080(2) , 148-56, (2005)
Full Text: HTML
Abstract
A rapid and simple method for confirmation of the diarrhetic shellfish poisons (DSP): okadaic acid (OA), dinophysistoxin-1 (DTX-1) and dinophysistoxin-2 (DTX-2) using fluorescence detection following derivatization with 9-chloromethylanthracene, has been established as an alternate to LC/MS. Exposure of the anthrylmethyl derivatives of OA, DTX-1 and DTX-2 to near UV light (300-400 nm) resulted in the loss of these compounds to below detection limits within 30 min, with a concurrent appearance of two additional compounds. Based on the mass spectral evidence, we propose that these newly formed compounds are the decarboxylation products of the derivatized diarrhetic shellfish poisons. UV radiation is, therefore, proposed as a rapid and simple confirmation technique for these DSP in mussel samples.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
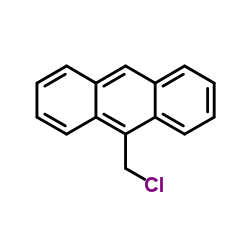 |
9-Chloromethylanthracene
CAS:24463-19-2 |
C15H11Cl |
|
Efficient and rapid template-directed nucleic acid copying u...
2009-10-14 [J. Am. Chem. Soc. 131(40) , 14560-70, (2009)] |
|
Methotrexate-loaded biodegradable polymeric micelles: prepar...
2005-08-01 [Colloids Surf. B Biointerfaces 44(2-3) , 104-9, (2005)] |
|
Liquid chromatographic determination of okadaic acid and din...
1996-01-19 [J. Chromatogr. A. 721(2) , 359-64, (1996)] |
|
Comparison of different fluorimetric HPLC methods for analys...
2003-12-01 [Anal. Bioanal. Chem 377(7-8) , 1202-6, (2003)] |
|
Quantitative analysis of monofluoroacetate in biological sam...
2007-09-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 857(1) , 53-8, (2007)] |