Accurate measurement of the exciton diffusion length in a conjugated polymer using a heterostructure with a side-chain cross-linked fullerene layer.
Denis E Markov, Emiel Amsterdam, Paul W M Blom, Alexander B Sieval, Jan C Hummelen
Index: J. Phys. Chem. A 109(24) , 5266-74, (2005)
Full Text: HTML
Abstract
Exciton diffusion and photoluminescence quenching in conjugated polymer/fullerene heterostructures are studied by time-resolved photoluminescence. It is observed that heterostructures consisting of a spin-coated poly(p-phenylene vinylene) (PPV)-based derivative and evaporated C60 are ill-defined because of diffusion of C60 into the polymer, leading to an overestimation of the exciton diffusion length. This artifact is resolved by the use of a novel, thermally side-chain polymerizing and cross-linking fullerene derivative (F2D) containing two diacetylene moieties, forming a completely immobilized electron acceptor layer. With this heterostructure test system, an exciton diffusion length of 5 +/- 1 nm is derived for this PPV derivative from time-integrated luminescence quenching data.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
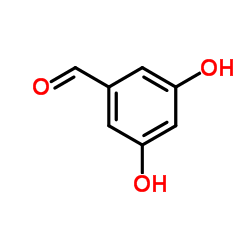 |
3,5-DIHYDROXYBENZALDEHYDE
CAS:26153-38-8 |
C7H6O3 |
|
Hepatocyte-targeting gene delivery using a lipoplex composed...
2014-10-01 [Bioorg. Med. Chem. 22(19) , 5212-9, (2014)] |
|
Health-Beneficial Phenolic Aldehyde in Antigonon leptopus Te...
2008-12-01 [Evid. Based. Complement. Alternat. Med. 2011 , 601249, (2012)] |
|
Utilization of Dioxygen by Carotenoid Cleavage Oxygenases.
2015-12-18 [J. Biol. Chem. 290 , 30212-23, (2015)] |
|
Stability-indicating HPLC Method for Simultaneous Determinat...
2011-01-01 [Indian J. Pharm. Sci. 73 , 46-56, (2011)] |
|
Enzymatic resolution and pharmacological activity of the ena...
[Bioorg. Med. Chem. Lett. 5(3) , 223-28, (1995)] |