Journal of Pharmaceutical Sciences
1987-07-01
Thermochemical investigations of associated solutions: 4. Calculation of carbazole-dibutyl ether association constants from measured solubility in binary solvent mixtures.
J W McCargar, W E Acree
Index: J. Pharm. Sci. 76(7) , 572-4, (1987)
Full Text: HTML
Abstract
Experimental solubilities are reported for anthracene and carbazole in binary dibutyl ether plus n-hexadecane and dibutyl ether plus squalane solvent mixtures at 25 degrees C. Results of these measurements, used in conjunction with the extended nearly ideal binary solvent (NIBS) model, enabled calculation of the carbazole-dibutyl ether association constant. The numerical value obtained was independent of the hydrocarbon cosolvent, and compared favorably with previously reported values based on carbazole solubilities in solvent mixtures containing much smaller alkane cosolvents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
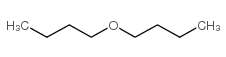 |
Di-n-butyl ether suppliers in China
CAS:142-96-1 |
C8H18O |
Related Articles:
More...
|
Novel unbreakable solid-phase microextraction fibers on stai...
2014-10-01 [Talanta 128 , 231-6, (2014)] |
|
Comparison between solid phase microextraction (SPME) and ho...
2014-09-01 [Talanta 127 , 59-67, (2014)] |
|
The study of binding of methyl tert-butyl ether to human tel...
2014-11-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 971 , 112-9, (2014)] |
|
Antibody-opsonized bacteria evoke an inflammatory dendritic ...
2015-02-15 [J. Immunol. 194(4) , 1856-66, (2015)] |
|
"Center punch" and "whole spot" bioanalysis of apixaban in h...
2015-04-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 988 , 66-74, (2015)] |