Chlorpropham induces mitochondrial dysfunction in rat hepatocytes.
Yoshio Nakagawa, Kazuo Nakajima, Toshinari Suzuki
Index: Toxicology 200(2-3) , 123-33, (2004)
Full Text: HTML
Abstract
The metabolism and action of chlorpropham (isopropyl N-(3-chlorophenyl)carbamate; CIPC, a post-harvest agent) and its metabolites were studied in freshly isolated rat hepatocytes and isolated rat hepatic mitochondria, respectively. The exposure of hepatocytes to CIPC caused a concentration (0.25-1.0 mM)- and time (0-3h)-dependent cell death accompanied by loss of cellular ATP and adenine nucleotides. CIPC at a weakly toxic level (0.5 mM) was metabolized to isopropyl N-(3-chloro-4-hydroxyphenyl)carbamate (4OH-CIPC) and subsequently to its glucuronide and sulfate conjugates (major metabolites) or alternatively to a minor metabolite 3-chloroaniline (3CA). The addition of SKF-525A (50 microM), an inhibitor of microsomal monooxygenase, enhanced the CIPC (0.5 mM)-induced cytotoxicity accompanied by loss of ATP and 4OH-CIPC and inhibited the decrease in the concentration of the parent compound. CIPC led to a strong decrease in cellular ATP content compared to its metabolites, 4OH-CIPC and 3CA. On the other hand, the exposure of isolated hepatic mitochondria to CIPC reduced State 3 respiration with a FAD-linked substrate (succinate plus rotenone) and/or with a NAD+ -linked substrate (pyruvate plus malate), whereas State 3 respiration with ascorbate plus tetramethyl-p-phenylendiamine (cytochrome oxidase-linked respiration) was not affected markedly by CIPC. Further, the addition of CIPC caused an increase in the rate of State 4 oxygen consumption, indicating an uncoupling effect, and a decrease in the rate of State 3 oxygen consumption in a concentration-dependent manner, respectively. In contrast, the addition of neither 4OH-CIPC nor 3CA markedly affected the rate of states 3 and/or 4 oxygen consumption. These results indicate that CIPC-induced cytotoxicity is mediated by the parent compound rather than by its metabolites such as 4OH-CIPC and 3CA, and that the toxicity is associated with a rapid depletion of ATP via impairment of mitochondrial function related to oxidative phosphorylation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
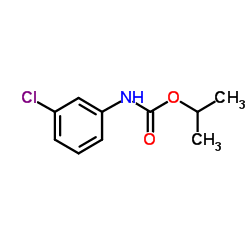 |
Chlorpropham
CAS:101-21-3 |
C10H12ClNO2 |
|
[Survey of pesticide residues in imported frozen vegetables ...
2011-01-01 [Shokuhin Eiseigaku Zasshi 52(2) , 121-9, (2011)] |
|
Mining biologically-active molecules for inhibitors of fatty...
2009-01-01 [Bioorg. Med. Chem. Lett. 19 , 6793-6, (2009)] |
|
The sprout inhibitors chlorpropham and 1,4-dimethylnaphthale...
2010-05-01 [Plant Mol. Biol. 73(1-2) , 181-9, (2010)] |
|
Rapid determination of aniline metabolites of chlorpropham i...
2005-08-01 [Electrophoresis 26(15) , 2991-8, (2005)] |
|
Planktonic versus biofilm catabolic communities: importance ...
2011-07-01 [Appl. Environ. Microbiol. 77(14) , 4728-35, (2011)] |