| Structure | Name/CAS No. | Articles |
|---|---|---|
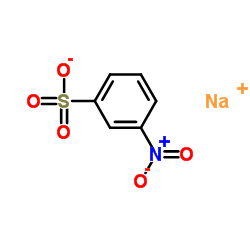 |
Sodium 3-nitrobenzenesulfonate
CAS:127-68-4 |
V Calderara, M Jekel, C Zaror
Index: Water Sci. Technol. 44(5) , 7-13, (2001)
Full Text: HTML
This paper describes the ozone oxidation kinetics of 1-naphthalene (1 NS), 1,5-naphthalene (1,5NDS), and 3-nitrobenzene (3NBS) sulphonic acid. The presence of hydroxyl radicals and their effect on the overall rate of reaction was studied. Second order kinetic constants of direct reactions were estimated at around 252 M(-1) s(-1), 41 M(-1) s(-1) and 22 M(-1) s(-1), for 1NS, 1,5NDS, and 3NBS sulphonic acids, respectively. At pH 3, the indirect reaction accounted for 2%, 15% and 4% of total primary oxidation of 1 NS, 1,5NBS, and 3NBS sulphonic acids, respectively. At pH 9, indirect reaction contribution increased to 73%, 84% and 48%, respectively. C4 compounds (maleic and fumaric acids), C2 (oxalic), C1 (formic) and sulphate were identified as oxidation by-products in all cases. TOC slowly decreased throughout ozonation, reaching around 40-60% and 60-70% reduction over 90 minutes at pH 7 and 3, respectively.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Sodium 3-nitrobenzenesulfonate
CAS:127-68-4 |
C6H4NNaO5S |
|
Degradation of the synthetic dye amaranth by the fungus Bjer...
2011-11-01 [Biodegradation 22(6) , 1239-45, (2011)] |
|
Fabrication of biocompatible and tumor-targeting hyaluronan ...
2014-05-01 [J. Pharm. Sci. 103(5) , 1529-37, (2014)] |
|
Discovery of a substituted 8-arylquinoline series of PDE4 in...
2005-12-01 [Bioorg. Med. Chem. Lett. 15(23) , 5241-6, (2005)] |
|
Asymmetric cooperative catalysis of strong Brønsted acid-pro...
2010-02-19 [Science 327(5968) , 986-90, (2010)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved