Enzymatic digestibility of peptides cross-linked by ionizing radiation.
M Dizdaroglu, E Gajewski, M G Simic
Index: Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 45(3) , 283-95, (1984)
Full Text: HTML
Abstract
p6gestibility by proteolytic enzymes of peptides cross-linked by ionizing radiation was investigated. Small peptides of alanine and phenylalanine were chosen as model compounds and aminopeptidases and carboxypeptidases were used as proteolytic enzymes. Peptides exposed to gamma-radiation in aqueous solution were analysed by high-performance liquid chromatography before and after hydrolysis by aminopeptidase M, leucine aminopeptidase, carboxypeptidase A and carboxypeptidase Y. The results obtained clearly demonstrate the different actions of these enzymes on cross-linked aliphatic and aromatic peptides. Peptide bonds of cross-linked dipeptides of alanine were completely resistant to enzymatic hydrolysis whereas the enzymes, except for carboxypeptidase Y, cleaved all peptide bonds of cross-linked peptides of phenylalanine. The actions of the enzymes on these particular compounds are discussed in detail.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
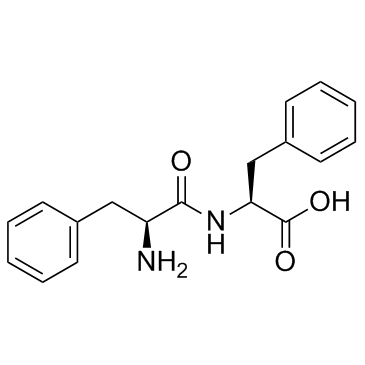 |
H-Phe-Phe-OH
CAS:2577-40-4 |
C18H20N2O3 |
|
Metabolomic profiling of serum in the progression of Alzheim...
2014-12-01 [Electrophoresis 35(23) , 3321-30, (2014)] |
|
Possible mechanism of uptake for several compounds in ionize...
1984-01-30 [Life Sci. 34(5) , 427-36, (1984)] |
|
pH-dependence of complexion constants and complex mobility i...
2001-09-01 [Electrophoresis 22(15) , 3163-70, (2001)] |
|
A symmetric inhibitor binds HIV-1 protease asymmetrically.
1993-01-26 [Biochemistry 32(3) , 937-47, (1993)] |
|
Carbohydrate binding sites in Candida albicans exo-β-1,3-glu...
2010-11-01 [FEBS J. 277(21) , 4549-61, (2010)] |