η1-Allylpalladium complexes with a tridentate PNP ligand with different phosphino groups.
Bruno Crociani, Simonetta Antonaroli, Paola Paoli, Patrizia Rossi
Index: Dalton Trans. 41(40) , 12490-500, (2012)
Full Text: HTML
Abstract
The iminodiphosphine 2-(PPh(2))C(6)H(4)-1-CH=NC(6)H(4)-2-(PPh(2)) (P-N-P') is used for the preparation of the complexes [Pd(η(1)-CHR(1)-CH=CR(2)R(3))(P-N-P')]BF(4) [R(1) = R(2) = R(3) = H: (1); R(1) = R(2) = Ph, R(3) = H: (2); R(1) = R(3) = H, R(2) = Ph: (3); R(1) = H, R(2) = R(3) = Me: (4)]. The P-N-P' tridentate coordination and the η(1)-allyl bonding mode in the solid are confirmed by the X-ray structural analysis of 1. In solution, the complexes 1 and 2 undergo an η(1)-η(3)-η(1) rearrangement at 298 K interconverting the bonding site of the allyl group. A five-coordinate structure with the phosphine ligands in the axial position is proposed for the η(3)-allyl intermediate. For the dynamic process, a ΔG(≠) value of 53.8 kJ mol(-1) is obtained from (1)H NMR data of 2. In 3 and 4, the allyl ligand is rigidly bound to the metal through the less substituted terminus, in line with the higher free energy content of the corresponding isomers: [Pd(η(1)-CHPh-CH=CH(2))(P-N-P')](+) +48.78 kJ mol(-1); [Pd(η(1)-CMe(2)-CH[double bond, length as m-dash]CH(2))(P-N-P')](+) +69.35 kJ mol(-1). The complexes react with secondary amines in the presence of fumaronitrile at different rates yielding allylamines and the palladium(0) derivative [Pd(η(2)-fn)(P-N-P')] (5). On the basis of charge distribution on the allylic carbon atoms and of steric factors, the difference in rate and the regioselectivity in the amination of 1-3 are better rationalized by a mechanism with nucleophilic attack at the η(3)-intermediate rather than by an S(N)2 mechanism with nucleophilic attack at the Pd-CHR(1) carbon atom. The high regioselectivity in the reaction of 4 with piperidine implies an S(N)2' mechanism with nucleophilic attack at the CMe(2) allyl carbon. A dynamic process occurs also for the 18-electron complex 5 consisting in a dissociation-association equilibrium of the olefin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
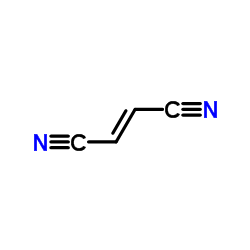 |
Fumaronitrile
CAS:764-42-1 |
C4H2N2 |
|
Effect of dosing vehicle on the toxicity and metabolism of u...
1995-01-01 [J. Appl. Toxicol. 15(5) , 411-20, (1995)] |
|
Ultrafast excited state dynamics of the perylene radical cat...
2006-06-22 [J. Phys. Chem. A 110(24) , 7547-53, (2006)] |
|
Bringing nitrilase sequences from databases to life: the sea...
2016-03-01 [Appl. Microbiol. Biotechnol. 100 , 2193-202, (2016)] |
|
The development of a novel strategy for the microbial treatm...
1995-06-01 [Biodegradation 6(2) , 93-107, (1995)] |
|
Ultrabright and ultrastable near-infrared dye nanoparticles ...
2012-11-01 [Biomaterials 33(31) , 7803-9, (2012)] |