Interactions of monovalent cations with sodium channels in squid axon. II. Modification of pharmacological inactivation gating.
J Z Yeh, G S Oxford
Index: J. Gen. Physiol. 85 , 603-620, (1985)
Full Text: HTML
Abstract
The time-, frequency-, and voltage-dependent blocking actions of several cationic drug molecules on open Na channels were investigated in voltage-clamped, internally perfused squid giant axons. The relative potencies and time courses of block by the agents (pancuronium [PC], octylguanidinium [C8G], QX-314, and 9-aminoacridine [9-AA]) were compared in different intracellular ionic solutions; specifically, the influences of internal Cs, tetramethylammonium (TMA), and Na ions on block were examined. TMA+ was found to inhibit the steady state block of open Na channels by all of the compounds. The time-dependent, inactivation-like decay of Na currents in pronase-treated axons perfused with either PC, 9-AA, or C8G was retarded by internal TMA+. The apparent dissociation constants (at zero voltage) for interaction between PC and 9-AA with their binding sites were increased when TMA+ was substituted for Cs+ in the internal solution. The steepness of the voltage dependence of 9-AA or PC block found with internal Cs+ solutions was greatly reduced by TMA+, resulting in estimates for the fractional electrical distance of the 9-AA binding site of 0.56 and 0.22 in Cs+ and TMA+, respectively. This change may reflect a shift from predominantly 9-AA block in the presence of Cs+ to predominantly TMA+ block. The depth, but not the rate, of frequency-dependent block by QX-314 and 9-AA is reduced by internal TMA+. In addition, recovery from frequency-dependent block is not altered. Elevation of internal Na produces effects on 9-AA block qualitatively similar to those seen with TMA+. The results are consistent with a scheme in which the open channel blocking drugs, TMA (and Na) ions, and the inactivation gate all compete for a site or for access to a site in the channel from the intracellular surface. In addition, TMA ions decrease the apparent blocking rates of other drugs in a manner analogous to their inhibition of the inactivation process. Multiple occupancy of Na channels and mutual exclusion of drug molecules may play a role in the complex gating behaviors seen under these conditions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
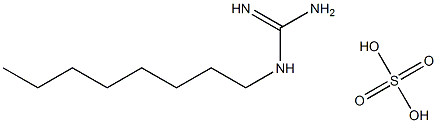 |
1-Octylguanidine hemisulfate
CAS:21409-35-8 |
CH3(CH2)7NHC(=NH)NH2·1/2H2SO4 |
|
Inactivation of mitochondrial permeability transition pore b...
2000-04-01 [J. Bioenerg. Biomembr. 32(2) , 193-8, (2000)] |
|
Environment-selective synergism using self-assembling cytoto...
1988-12-01 [Biochem. Pharmacol. 37(23) , 4505-12, (1988)] |
|
Myocardial protective effect of octylguanidine against the d...
2005-01-01 [Mol. Cell Biochem. 269(1-2) , 19-26, (2005)] |
|
Effects of octylguanidine on heart activity: 2. Mechanical c...
1980-03-30 [Boll. Soc. Ital. Biol. Sper. 56(6) , 625-9, (1980)] |
|
Effects of octylguanidine on heart activity: 1. Electrical c...
1980-03-30 [Boll. Soc. Ital. Biol. Sper. 56(6) , 619-24, (1980)] |