12-Deoxyphorbol-13-O-phenylacetate-20-acetate is not protein kinase C-beta isozyme-selective in vivo.
S C Kiley, A R Olivier, P C Gordge, W J Ryves, F J Evans, D K Ways, P J Parker
Index: Carcinogenesis 15(2) , 319-24, (1994)
Full Text: HTML
Abstract
The phorbol ester, 12-deoxyphorbol-13-O-phenylacetate-20-acetate (DOPPA) has been shown to activate specifically the protein kinase C (PKC)-beta 1 isozyme in vitro (1). We have investigated the potential of DOPPA as a PKC-beta 1/2 isozyme-specific agonist in intact cells, employing U937 cells, which express beta 1/2, epsilon and zeta PKC and in Swiss 3T3 cells which lack PKC-beta 1/2 but express alpha, delta, epsilon and zeta PKC. Immunoblot analysis with isozyme-specific antibodies indicated that DOPPA can mediate the subcellular redistribution and down-modulation of all endogenous PKC isozymes (except PKC-zeta) in both U937 and Swiss 3T3 cells. Prolonged treatment (> 6 h) of cultures in down-modulation studies is complicated by the metabolism of DOPPA to 12-deoxyphorbol-13-phenylacetate (DOPP), a compound which activates all PKC isozymes tested in vitro (Ryves, W. J., et al. (1991) FEBS Lett., 288, 5-9). Nevertheless, because DOPPA induced rapid and dose-dependent phosphorylation of p80 in cells which do not express PCK-beta, p80 phosphorylation in Swiss 3T3 cells indicates that DOPPA can activate a non-beta PKC in vivo. The data suggest that DOPPA cannot be used as a PKC-beta-selective agonist in intact cell studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
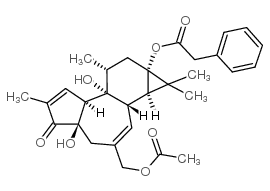 |
12-deoxyphorbol 13-phenylacetate 20-acetate
CAS:54662-30-5 |
C30H36O7 |
|
Phosphorylation of connexin43 and inhibition of gap junction...
2001-02-01 [Carcinogenesis 22(2) , 221-31, (2001)] |
|
Protein kinase C modulates insulin action in human skeletal ...
2000-03-01 [Am. J. Physiol. Endocrinol. Metab. 278(3) , E553-62, (2000)] |
|
Rapid loss of blood-brain barrier P-glycoprotein activity th...
2010-09-01 [J. Cereb. Blood Flow Metab. 30(9) , 1593-7, (2010)] |
|
Inhibition of erythema induced by pro-inflammatory esters of...
1981-01-01 [Acta Pharmacol. Toxicol. (Copenh.) 48(1) , 47-52, (1981)] |
|
Protein kinase Cdelta-dependent induction of manganese super...
1998-12-18 [J. Biol. Chem. 273(51) , 34639-45, (1998)] |