Molecules
2007-01-01
Synthesis of some new anils: part 1. Reaction of 2-hydroxy-benzaldehyde and 2-hydroxynaphthaldehyde with 2-aminopyridene and 2-aminopyrazine.
Abdullah M Asiri, Khadija O Badahdah
Index: Molecules 12(8) , 1796-804, (2007)
Full Text: HTML
Abstract
New Schiff bases derived from 2-aminopyridene and 2-aminopyrazine have been synthesized. The UV-Visible spectra of the compounds have been investigated in acetonitrile and toluene. The compounds were in tautomeric equilibrium (enol-imine O- H...N, keto-amine O...H-N forms) in polar and nonpolar solvents. For some derivatives the keto-amine form was observed in both toluene and acetonitrile. 1H-NMR and IR results showed that all Schiff bases studied favor the enol-imine form over the keto form in a weakly polar solvent such as deuterochloroform.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
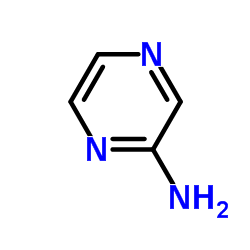 |
Aminopyrazine
CAS:5049-61-6 |
C4H5N3 |
Related Articles:
More...
|
Distribution study of atorvastatin and its metabolites in ra...
2014-07-01 [Anal. Bioanal. Chem 406(19) , 4601-10, (2014)] |
|
In vitro and in vivo activities of 2-aminopyrazines and 2-am...
2013-02-01 [Antimicrob. Agents Chemother. 57(2) , 1012-8, (2013)] |
|
Chemistry around imidazopyrazine and ibuprofen: discovery of...
2010-09-01 [Eur. J. Med. Chem. 45 , 3564-74, (2010)] |
|
Identification of low molecular weight carbohydrates employi...
2008-07-01 [Rapid Commun. Mass Spectrom. 22 , 2185-2194, (2008)] |
|
Catechol derivatives of aminopyrazine and cell protection ag...
2001-04-01 [Bioorg. Med. Chem. 9(4) , 1037-44, (2001)] |