Calorimetric and diffractometric evidence for the sequential crystallization of buffer components and the consequential pH swing in frozen solutions.
Prakash Sundaramurthi, Evgenyi Shalaev, Raj Suryanarayanan
Index: J. Phys. Chem. B 114 , 4915-23, (2010)
Full Text: HTML
Abstract
Sequential crystallization of succinate buffer components in the frozen solution has been studied by differential scanning calorimetry and X-ray diffractometry (both laboratory and synchrotron sources). The consequential pH shifts were monitored using a low-temperature electrode. When a solution buffered to pH < pK(a)(2) was cooled from room temperature (RT), the freeze-concentrate pH first increased and then decreased. This was attributed to the sequential crystallization of succinic acid, monosodium succinate, and finally disodium succinate. When buffered to pH > pK(a)(2), the freeze-concentrate pH first decreased and then increased due to the sequential crystallization of the basic (disodium succinate) followed by the acidic (monosodium succinate and succinic acid) buffer components. XRD provided direct evidence of the crystallization events in the frozen buffer solutions, including the formation of disodium succinate hexahydrate [Na(2)(CH(2)COO)(2).6H(2)O]. When the frozen solution was warmed in a differential scanning calorimeter, multiple endotherms attributable to the melting of buffer components and ice were observed. When the frozen solutions were dried under reduced pressure, ice sublimation was followed by dehydration of the crystalline hexahydrate to a poorly crystalline anhydrate. However, crystalline succinic acid and monosodium succinate were retained in the final lyophiles. The pH and the buffer salt concentration of the prelyo solution influenced the crystalline salt content in the final lyophile. The direction and magnitude of the pH shift in the frozen solution depended on both the initial pH and the buffer concentration. In light of the pH-sensitive nature of a significant fraction of pharmaceuticals (especially proteins), extreme care is needed in both the buffer selection and its concentration.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
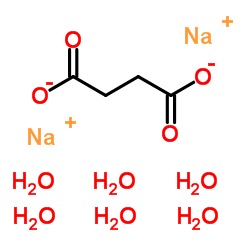 |
SODIUM SUCCINATE HEXAHYDRATE
CAS:6106-21-4 |
C4H16Na2O10 |
|
Oxidative damage of rat liver mitochondria during exposure t...
2013-06-21 [Life Sci. 92(23) , 1110-7, (2013)] |
|
Acquired resistance to gemcitabine and cross-resistance in h...
2015-01-01 [Anticancer Drugs 26(1) , 90-100, (2014)] |
|
Conditionally replicative adenoviral vectors for imaging the...
2013-08-01 [Cancer Sci. 104(8) , 1083-90, (2013)] |
|
Insights into the mechanism of Pseudomonas dacunhae aspartat...
2010-06-22 [Biochemistry 49 , 5066-73, (2010)] |
|
Ecological divergence of a novel group of Chloroflexus strai...
2013-02-01 [Appl. Environ. Microbiol. 79(4) , 1353-8, (2013)] |