| Structure | Name/CAS No. | Articles |
|---|---|---|
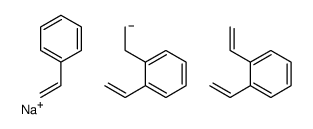 |
dowex(r) hcr-w2
CAS:69011-22-9 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
dowex(r) hcr-w2
CAS:69011-22-9 |