| Structure | Name/CAS No. | Articles |
|---|---|---|
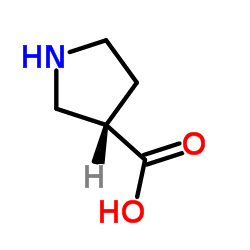 |
(S)-pyrrolidine-3-carboxylic acid
CAS:72580-53-1 |
|
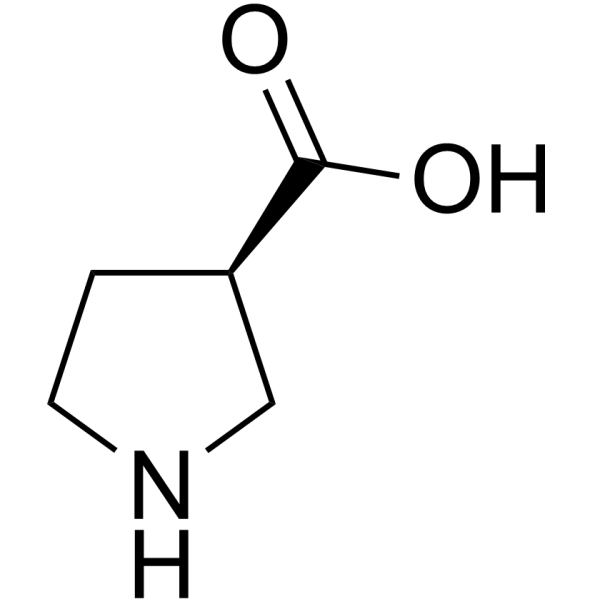 |
(R)-pyrrolidine-3-carboxylic acid
CAS:72580-54-2 |