| Structure | Name/CAS No. | Articles |
|---|---|---|
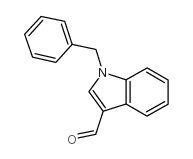 |
1H-Indole-3-carboxaldehyde,1-(phenylmethyl)
CAS:10511-51-0 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
1H-Indole-3-carboxaldehyde,1-(phenylmethyl)
CAS:10511-51-0 |