Elementary peptide motifs in the gas phase: FTIR aggregation study of formamide, acetamide, N-methylformamide, and N-methylacetamide.
Merwe Albrecht, Corey A Rice, Martin A Suhm
Index: J. Phys. Chem. A 112(33) , 7530-42, (2008)
Full Text: HTML
Abstract
Cold, isolated peptide model compounds and their aggregates are generated in pulsed supersonic jet expansions and detected by FTIR spectroscopy in the amide-A region, complemented by amide-I spectra. The most stable, symmetric dimer of formamide is unambiguously assigned in the gas phase for the first time, also by comparison to the analogous acetamide dimer. Efficient quenching of a hot-state Fermi resonance by cooling of the dimers is invoked. As the preferred relative orientation of the C=O and N-H groups in N-methylated formamide and acetamide is trans, these compounds show a fundamentally different dimerization pattern. Their most stable dimers, which would be analogous to those of formamide and acetamide, remain undetected as a consequence of kinetic control in the jet. Accurate benchmark quantities for multidimensional vibrational treatments of these peptide models are derived, and the influence of methyl groups on the N-H stretching dynamics is discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
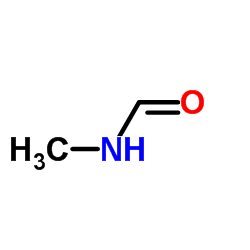 |
N-methylformamide
CAS:123-39-7 |
C2H5NO |
|
Surface-enhanced resonance Raman scattering nanostars for hi...
2015-01-21 [Sci. Transl. Med. 7(271) , 271ra7, (2015)] |
|
Mitochondrial DNA alterations in blood of the humans exposed...
2007-02-20 [Chem. Biol. Interact. 165(3) , 211-9, (2007)] |
|
Amidic and acetonic cryoprotectants improve cryopreservation...
2012-01-01 [Cryo. Letters 33(3) , 202-13, (2012)] |
|
N-methylformamide: antitumour activity and metabolism in mic...
1982-06-01 [Br. J. Cancer 45(6) , 843-50, (1982)] |
|
Use of N-methylformamide as a solvent in indium-promoted Bar...
2009-08-21 [J. Org. Chem. 74(16) , 5861-70, (2009)] |