A new strategy for the synthesis of cyclopeptides containing diaminoglutaric acid.
T Bayer, C Riemer, H Kessler
Index: J. Pept. Sci. 7(5) , 250-61, (2001)
Full Text: HTML
Abstract
A new synthesis of orthogonally protected diaminoglutaric acid containing peptides using the Ugi four component condensation is presented. To demonstrate that this method is useful to replace cystine by diaminoglutaric acid in biologically interesting peptides, we built up two cyclic somatostatin analogues deriving from Sandostatin and from TT-232. A photolytically cleavable amine derivative of the nitroveratryl type is used for the Ugi four component condensation. Because of a racemic build up of the new stereocentre of the diaminoglutaric acid, and racemization of the isonitrile component, four diastereomeric peptides resulted that were separated by HPLC. The stereochemistry of the cyclopeptides could be easily and unambiguously assigned by chiral gas chromatography and a reference sample of enantiomerically pure (2S,4S)-diaminoglutaric acid.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
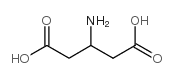 |
3-AMINOPENTANEDIOIC ACID
CAS:1948-48-7 |
C5H9NO4 |
|
Adsorption and polymerization of amino acids on mineral surf...
2008-06-01 [Orig. Life Evol. Biosph. 38 , 211-242, (2008)] |
|
Polymerization on the rocks: beta-amino acids and arginine.
1998-06-01 [Orig. Life Evol. Biosph. 28 , 245-257, (1998)] |
|
beta-Glutamate as a substrate for glutamine synthetase.
2001-10-01 [Appl. Environ. Microbiol. 67 , 4458-4463, (2001)] |
|
On the configuration of the receptors for excitatory amino a...
1982-06-01 [Neuropharmacology 21(6) , 549-54, (1982)] |
|
Occurrence of beta-glutamate, a novel osmolyte, in marine me...
1990-05-01 [Appl. Environ. Microbiol. 56(5) , 1504-8, (1990)] |