Structural and mechanistic analysis through electronic spectra: aqueous hyponitrite radical (N2O2-) and nitrosyl hyponitrite anion (N3O3-).
Marat Valiev, Sergei V Lymar
Index: J. Phys. Chem. A 115(43) , 12004-10, (2011)
Full Text: HTML
Abstract
Aqueous hyponitrite radical (N(2)O(2)(-)) and nitrosyl hyponitrite anion (N(3)O(3)(-)) are important intermediates in the reductive chemistry of NO. The structures and absorption spectra of various hydrated isomers of these compounds were investigated in this work using high-level quantum mechanical calculations combined with the explicit classical description of the aqueous environment. For N(2)O(2)(-), comparison of the calculated spectra and energetics with the experimental data reveals that (1) upon the one-electron oxidation of trans-hyponitrite (ON═NO(2-)), the trans configuration of the resulting ON═NO(-) radical is preserved; (2) although cis- and trans-ON═NO(-) are energetically nearly equivalent, the barrier for the trans-cis isomerization is prohibitively high because of the partial double character of the NN bond; (3) the calculations confirm that the UV spectrum of ONNO(-) was misinterpreted in the earlier pulse radiolysis work, and its more recent revision has been justified. For the N(3)O(3)(-) ion, the symmetric isomer [Formula: see text] is the dominant observable species, and the asymmetric isomer [Formula: see text] contributes insignificantly to the experimental spectrum. Coherent analysis of the calculated and experimental data suggests a reinterpretation of the N(2)O(2)(-) + NO reaction mechanism according to which the reaction evenly bifurcates to yield both the symmetric and asymmetric isomers of N(3)O(3)(-). While the latter isomer rapidly decomposes to the final NO(2)(-) + N(2)O products, the former isomer is stable toward this decomposition, but its formation is reversible with the homolysis equilibrium constant K(hom) = 2.2 × 10(-7) M. Collectively, these results demonstrate that advanced theoretical modeling can be of significant benefit in structural and mechanistic analysis on the basis of the electronic spectra of aqueous transients.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
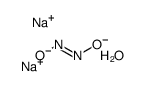 |
SODIUM TRANS-HYPONITRITE HYDRATE
CAS:60884-94-8 |
H2N2Na2O3 |
|
The use of cyclic nitroxide radicals as HNO scavengers.
2013-01-01 [J. Inorg. Biochem. 118 , 155-61, (2013)] |
|
Linkage isomerization in heme-NOx compounds: understanding N...
2010-07-19 [Inorg. Chem. 49(14) , 6253-66, (2010)] |
|
The structure of the hyponitrite species in a heme Fe-Cu bin...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46(13) , 2210-4, (2007)] |
|
Hyponitrite radical, a stable adduct of nitric oxide and nit...
2004-01-28 [J. Am. Chem. Soc. 126(3) , 891-9, (2004)] |
|
A stable hyponitrite-bridged iron porphyrin complex.
2009-02-25 [J. Am. Chem. Soc. 131(7) , 2460-1, (2009)] |