Selective methodologies for the synthesis of biologically active piperidinic compounds.
Janine Cossy
Index: Chem. Rec. 5(2) , 70-80, (2005)
Full Text: HTML
Abstract
The synthesis of optically active substituted piperidines has been achieved by using four different methodologies. The first one is an intramolecular nucleophilic displacement of activated alcohol moieties that was used to build up the piperidine ring of (-)-prosophylline and (-)-slaframine, and the second one is a ring-closing metathesis of unsaturated amines which was employed in the synthesis of (+)-sedamine and 4a,5-dihydrostreptazoline. The third methodology is the alpha-functionalization of N-Boc piperidines which was particularly useful in the synthesis of argatroban, and the fourth one is a ring expansion of prolinols to 3-chloropiperidines or 3-hydroxypiperidines which was utilized to synthesize (-)-paroxetine, (-)-pseudoconhydrine, the piperidine ring of (-)-velbanamine and (+)-zamifenacin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
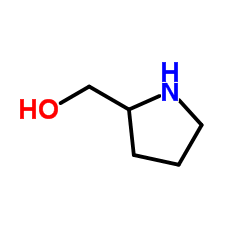 |
L-Pro-ol
CAS:23356-96-9 |
C5H11NO |
|
Three-dimensional quantitative structure-activity relationsh...
2011-11-01 [Bioorg. Med. Chem. 19 , 6409-18, (2011)] |
|
Chiral Diphenylprolinol TES ether promoted conjugate additio...
2007-03-15 [Org. Lett. 9(6) , 965-8, (2007)] |
|
Asymmetric alkynylation of aldehydes with propiolates withou...
2011-06-21 [Org. Biomol. Chem. 9(12) , 4425-8, (2011)] |
|
CBS reductions with a fluorous prolinol immobilized in a hyd...
2008-03-06 [Org. Lett. 10(5) , 749-52, (2008)] |
|
Access to optically active 3-azido- and 3-aminopiperidine de...
2011-08-19 [Org. Lett. 13(16) , 4442-5, (2011)] |