Organic & Biomolecular Chemistry
1997-07-22
Efficient proline and prolinol ether mediated 3-component synthesis of 3- and 3,4-substituted chromenone derivatives.
C Y Shiau, M F Byford, R T Aplin, J E Baldwin, C J Schofield
Index: Org. Biomol. Chem. 10(30) , 6201-10, (2012)
Full Text: HTML
Abstract
A highly efficient route for the synthesis of valuable 3,4-substituted chromenone derivatives by the reaction of 1,3-diketones with aldehydes in the presence of l-proline was developed. The reactions take advantage of readily available starting materials and follow a Knoevenagel condensation/Michael addition/hemiacetalization domino process. Chiral 3-substituted chromenones are obtained with high enantioselectivities when a chiral diarylprolinol TMS-ether is applied in the reaction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
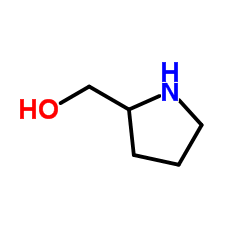 |
L-Pro-ol
CAS:23356-96-9 |
C5H11NO |
Related Articles:
More...
|
Three-dimensional quantitative structure-activity relationsh...
2011-11-01 [Bioorg. Med. Chem. 19 , 6409-18, (2011)] |
|
Chiral Diphenylprolinol TES ether promoted conjugate additio...
2007-03-15 [Org. Lett. 9(6) , 965-8, (2007)] |
|
Asymmetric alkynylation of aldehydes with propiolates withou...
2011-06-21 [Org. Biomol. Chem. 9(12) , 4425-8, (2011)] |
|
CBS reductions with a fluorous prolinol immobilized in a hyd...
2008-03-06 [Org. Lett. 10(5) , 749-52, (2008)] |
|
Access to optically active 3-azido- and 3-aminopiperidine de...
2011-08-19 [Org. Lett. 13(16) , 4442-5, (2011)] |