Infrared intensity in dielectric media by an electrostatic model.
Isao Kanesaka
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 60(1-2) , 297-302, (2004)
Full Text: HTML
Abstract
A change in an infrared intensity in dielectric media is treated by an electrostatic model. The basic model is originally formalized for a dipolar liquid. The model is satisfactorily applied to the infrared intensity of the C-H stretching of chloroform, which changes 22 times large in the liquid state at -43 degrees C as in the gaseous state. A change in the infrared intensity in lithium ammonium tartrate, where a ferroelectric phase transition takes place, is analyzed on the basis of a local polarization above T(c) or a spontaneous polarization below T(c), deducing important quantities on a phase transition. A difference in the infrared intensity of the C-Br stretching of 1,10-dibromodecane between the urea clathrate and the crystalline state is analyzed by evaluating electric fields due to bond moments and oscillating dipoles. These analyses confirm the mechanism of the change in the absolute infrared intensity, which originates from an electrostatic interaction with an electric field applied to a molecule or a functional group closely related to a normal mode.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
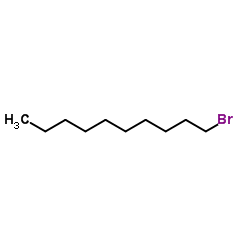 |
1-Bromodecane
CAS:112-29-8 |
C10H21Br |
|
Gas chromatography with parallel hard and soft ionization ma...
2015-01-15 [Rapid Commun. Mass Spectrom. 29(1) , 91-9, (2014)] |
|
Self-diffusion and interactions in mixtures of imidazolium b...
2015-07-01 [Magn. Reson. Chem. 53 , 493-7, (2015)] |
|
Tartaric acid-based amphiphilic macromolecules with ether li...
2015-06-01 [Biomaterials 53 , 32-9, (2015)] |
|
Comparative study on the biodegradability of morpholinium he...
2015-07-01 [Biodegradation 26 , 327-40, (2015)] |
|
Synthesis of surfactants based on pentaerythritol. I. Cation...
2009-10-16 [J. Org. Chem. 74(20) , 7762-73, (2009)] |