Bioorganic & Medicinal Chemistry Letters
2003-10-06
Xylylated dimers of putrescine and polyamines: influence of the polyamine backbone on spermidine transport inhibition.
Laurence Covassin, Michel Desjardins, Denis Soulet, René Charest-Gaudreault, Marie Audette, Richard Poulin
Index: Bioorg. Med. Chem. Lett. 13(19) , 3267-71, (2003)
Full Text: HTML
Abstract
Dimeric norspermidine and spermidine derivatives are strong competitive inhibitors of polyamine transport. A xylyl tether was used for the dimerization of various triamines and spermine via a secondary amino group, and of putrescine via an ether or an amino group. Dimerization of putrescine moieties potentiates their ability to compete against spermidine transport to a much greater extent than for triamine dimers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
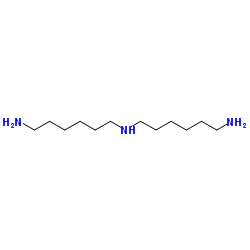 |
Bis(hexamethylene)triamine
CAS:143-23-7 |
C12H29N3 |
Related Articles:
More...
|
Asymmetric flow field-flow fractionation coupled to inductiv...
2015-11-27 [J. Chromatogr. A. 1422 , 247-52, (2015)] |
|
Dissociation between effects of polyamines on mitochondrial ...
1996-04-01 [Biochem. Mol. Biol. Int. 38(5) , 1003-11, (1996)] |
|
Polar/apolar chemical inducers of differentiation of transfo...
1989-08-01 [Proc. Natl. Acad. Sci. U. S. A. 86(16) , 6358-62, (1989)] |
|
Guest-directed supramolecular architectures of {W36} polyoxo...
2009-10-05 [Chem. Asian J. 4(10) , 1612-1618, (2009)] |
|
Development of high dispersed TiO2 paste for transparent scr...
[J. Nano. Res. 15(1) , 1-6, (2013)] |