Computational study of solvent effects on the molecular self-assembly of tetrolic acid in solution and implications for the polymorph formed from crystallization.
Jie Chen, Bernhardt L Trout
Index: J. Phys. Chem. B 112(26) , 7794-802, (2008)
Full Text: HTML
Abstract
The solution behavior of a model compound, tetrolic acid (TTA), is studied via molecular dynamics simulations in four organic solvents. The results suggest that strong interactions between TTA and solvent molecules (ethanol or dioxane) prevent the formation of carboxylic acid dimers in solution and thus promote the crystallization of TTA in a catemer-based form or a solvate form. Weak interactions, however, between TTA and solvent molecules (carbon tetrachloride or chloroform) facilitate the formation of carboxylic acid dimers in solution and thus promote the crystallization of a dimer-based crystal. Detailed solvent structure plays an important role in determining the relative stability of various growth synthons in solution and also the barriers along the pathway connecting them. This work illustrates the potential of molecular simulations to aid in the rational selection of solvents to obtain desired polymorphs during crystallization.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
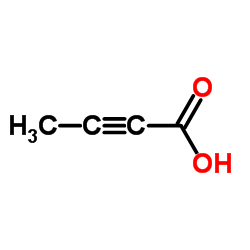 |
2-Butynoic acid
CAS:590-93-2 |
C4H4O2 |
|
An Acetate-Specific GPCR, FFAR2, Regulates Insulin Secretion...
2015-07-01 [Mol. Endocrinol. 29 , 1055-66, (2015)] |
|
Spatial and seasonal trends in biogenic secondary organic ae...
2008-07-15 [Environ. Sci. Technol. 42(14) , 5171-6, (2008)] |
|
Use of some novel alternative electron sinks to inhibit rumi...
2003-01-01 [Reprod. Nutr. Dev. 43(2) , 189-202, (2003)] |
|
Linking solution chemistry to crystal nucleation: the case o...
2005-03-28 [Chem. Commun. (Camb.) (12) , 1531-3, (2005)] |
|
Biological assessments of experimental cavity cleansers: cor...
1982-08-01 [J. Dent. Res. 61(8) , 967-72, (1982)] |