Pharmacokinetics of dibenzylamine administered in a sustained drug delivery system with cefazolin.
M B Calvo, J L Pedraz, A Domínguez-Gil
Index: Biopharm. Drug Dispos. 11(9) , 797-806, (1990)
Full Text: HTML
Abstract
The pharmacokinetics of dibenzylamine administered in a sustained drug delivery system with cefazolin was studied after i.m. administration of a dose of 1250 mg to healthy volunteers. The serum and urine levels of dibenzylamine were determined by a GLC technique using a specific nitrogen-phosphorus detector. Characterization of the kinetic parameters was performed by applying compartmental and non-compartmental analysis. Dibenzylamine was found to reach concentrations close to 300 ng ml-1 approximately 5 h after administration. The elimination constant had a value of 0.832 +/- 0.821 h-1 (mean +/- S.D.), which is higher than the release constant of the derivative (0.109 +/- 0.072 h-1) (mean +/- S.D.). These results show that release of dibenzylamine may be considered the limiting kinetic process, which governs the elimination of the product from the organism. Only a small amount of dibenzylamine is excreted in urine unchanged 3.43 +/- 3.28 per cent (mean +/- S.D.). Using the pharmacokinetic parameters calculated for dibenzylamine, a prediction has been made of the concentrations reached in a multiple dosage regimen after administration of a dose of 1250 mg every 24 h. The accumulation factor was 1.09.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
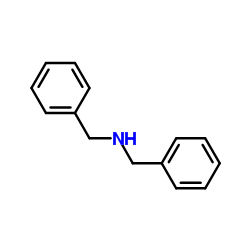 |
Dibenzylamine
CAS:103-49-1 |
C14H15N |
|
Spectrophotometric determination of folic acid in pharmaceut...
2002-08-15 [Anal. Biochem. 307(2) , 316-21, (2002)] |
|
Diastereoselective construction of syn-α-oxyamines via three...
2012-10-07 [Org. Biomol. Chem. 10(37) , 7536-44, (2012)] |
|
Kinetic study on the nitrosation of dibenzylamine in a model...
1994-11-01 [Food Chem. Toxicol. 32(11) , 1015-9, (1994)] |
|
Determination of imipramine in presence of iminodibenzyl and...
2003-11-24 [J. Pharm. Biomed. Anal. 33(4) , 775-82, (2003)] |
|
The discovery of an orally efficacious positive allosteric m...
2010-09-15 [Bioorg. Med. Chem. Lett. 20(18) , 5544-7, (2010)] |