Simultaneous determination of diloxanide furoate and metronidazole in presence of diloxanide furoate degradation products.
Samah S Abbas, Nour E Wagieh, Mohamed Abdelkawy, Maha M Abdelrahman
Index: J. AOAC Int. 94(5) , 1427-39, (2011)
Full Text: HTML
Abstract
Three methods are presented for the simultaneous determination of diloxanide furoate (DLX) and metronidazole (MTR), used for their antiprotozoal and antiamoebic effect, in the presence of DLX alkaline degradates and in pharmaceutical formulations, without previous separation. The first method is chemometric-assisted spectrophotometry, in which principal component regression and partial least squares were applied. These two approaches were successfully applied to quantify each drug in the mixture using the information included in, the absorption spectra in the range of 225-320 nm. The second method is TLC-densitometry, in which the binary mixture and degradates were separated on silica gel plates using a chloroform-acetone-glacial acetic acid (9.5 + 0.5 + 0.07, v/v/v) mobile phase and the bands were scanned at 254 nm. The last method is HPLC, in which DLX, MTR, and degradates were separated using the mobile phase acetonitrile-0.05 M dibasic potassium phosphate (25 + 75, v/v), adjusted to pH 4 with orthophosphoric acid, at a flow rate of 1 mL/min, on a C18 analytical column. Detection was at 254 nm. The proposed methods were successfully applied for the analysis of DLX and MTR in pharmaceutical formulations, and the results were statistically compared with a reported spectrophotometric method.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
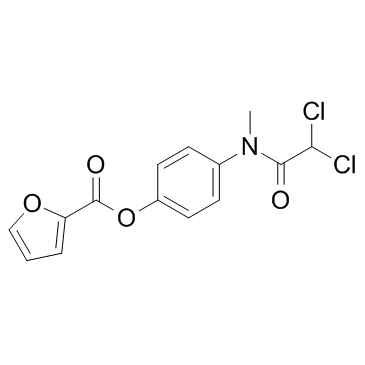 |
Diloxanide furoate
CAS:3736-81-0 |
C14H11Cl2NO4 |
|
Kinetic spectrophotometric H-point standard addition method ...
2013-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 114 , 592-8, (2013)] |
|
Validated chromatographic and spectrophotometric methods for...
2013-01-01 [Pak. J. Pharm. Sci. 26(1) , 175-83, (2013)] |
|
A misguided 'pill in the pocket' approach with flecainide le...
2012-01-01 [BMJ Case Rep. 2012 , doi:10.1136/bcr-2012-006868, (2012)] |
|
Luminal agents for invasive amebiasis: nice or necessary?
1993-06-01 [Am. J. Gastroenterol. 88(6) , 964-5, (1993)] |
|
In vitro evaluation of the suppressive effect of chitosan/po...
2012-08-30 [Eur. J. Pharm. Sci. 47(1) , 215-27, (2012)] |